Chemistry Essentials for Dummies
Chapter 7. Chemical Reactions
Knowing Chemical Equilibrium Backwards and Forwards
A dynamic chemical equilibrium is established when two exactly opposite chemical reactions are occurring at the same place, at the same time, with the same rates (speed) of reaction. I call this example a dynamic chemical equilibrium, because when the reactions reach equilibrium, things don’t just stop — the reactants still react to form the products, and those substances react to form the original reactants.
In this section, I explain how reactions reach equilibrium. I also introduce the equilibrium constant, which helps you find out how much product and reactant you have when the reaction is at equilibrium.
Matching rates of change in the Haber process
My favorite reaction is the Haber process, the synthesis of ammonia from nitrogen and hydrogen gases. After balancing the reaction (see the section “Balancing the Haber process,” earlier in this chapter), you end up with
![]()
Written this way, the reaction says that hydrogen and nitrogen react to form ammonia — and this keeps on happening until you use up one or both of the reactants. But this isn’t quite true.
If this reaction occurs in a closed container (which it has to, with everything being gases), then the nitrogen and hydrogen react and ammonia is formed — but some of the ammonia soon starts to decompose into nitrogen and hydrogen, like this:
![]()
In the container, then, you actually have two exactly opposite reactions occurring — nitrogen and hydrogen combine to give ammonia, and ammonia decomposes to give nitrogen and hydrogen.
Instead of showing the two separate reactions, you can show one reaction and use a double arrow like this:
![]()
You put the nitrogen and hydrogen on the left because that’s what you initially put into the reaction container. The reaction is exothermic (it gives off heat), so I’m showing heat as a product of the reaction on the right.
Now these two reactions occur at different speeds, but sooner or later, the two speeds become the same, and the relative amounts of nitrogen, hydrogen, and ammonia become constant. This is an example of a chemical equilibrium. At any given time in the Haber process, you have nitrogen and hydrogen reacting to form ammonia and ammonia decomposing to form nitrogen and hydrogen. When the system reaches equilibrium, the amounts of all chemical species become constant but not necessarily the same.
Constants: Comparing amounts of products and reactants
Sometimes there’s a lot of product (chemical species on the right-hand side of the double arrow) when the reaction reaches equilibrium, and sometimes there’s very little. You can tell the relative amounts of reactants and products at equilibrium if you know the equilibrium constant for the reaction. Look at a hypothetical equilibrium reaction:
![]()
The capital letters stand for the chemical species, and the small letters represent the coefficients in the balanced chemical equation. The equilibrium constant (represented as Keq) is mathematically defined as
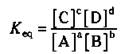
The numerator contains the product of the two chemical species on the right-hand side of the equation, with each chemical species raised to the power of its coefficient in the balanced chemical equation. The denominator is the same, but you use the chemical species on the left-hand side of the equation. (It’s not important right now, but those brackets stand for the molar concentration. You can find out what that is in Chapter 10.) Note that sometimes chemists use the Kc notation instead of the Keq form.
TIP. The numerical value of the equilibrium constant gives you a clue about the relative amounts of products and reactants. The larger the value of the equilibrium constant (Keq), the more products are present at equilibrium. If, for example, you have a reaction that has an equilibrium constant of 0.001 at room temperature and 0.1 at 100°C, you can say that you’ll have much more product at the higher temperature.
Now I happen to know that the Keq for the Haber process (the ammonia synthesis) is 3.5 x 108 at room temperature. This large value indicates that, at equilibrium, there’s a lot of ammonia produced from the nitrogen and hydrogen, but there’s still hydrogen and nitrogen left at equilibrium.