Chemistry Essentials for Dummies
Chapter 7. Chemical Reactions
Seeing How Catalysts Speed Up Reactions
Catalysts speed up reactions by lowering the activation energy of a reaction. They do this in one of two ways:
✓ Providing a surface and orientation that makes a reactant more likely to hit the right part of another reactant to break/make a bond
✓ Providing an alternative mechanism (series of steps for the reaction to go through) that has a lower activation energy
Heterogeneous catalysis: Giving reactants a better target
A heterogeneous catalyst ties one molecule to a surface, putting the molecule in an orientation that makes another reactant more likely to hit the reactive site. Suppose, for instance, you have the following generalized reaction (which I introduce earlier in “Collision Theory: How Reactions Occur”):
![]()
Reactant C must hit the reactive site on the A end of molecule A-B in order to break the A-B bond and form the C-A bond shown in the equation. The probability of the collision occurring in the proper orientation is pretty much driven by chance. The reactants are moving around, running into each other, and sooner or later the collision may occur at the reactive site. But what would happen if you could tie the A-B molecule down with the A end exposed? It’d be much easier and more probable for C to hit A with this scenario.
The catalyst that does this tying down is called heterogeneous because it’s in a different phase than the reactants. This catalyst is commonly a finely divided solid metal or metal oxide, and the reactants are gases or in solution. This heterogeneous catalyst tends to attract one part of a reactant molecule due to rather complex interactions that aren’t fully understood. After the reaction takes place, the forces that bound the B part of the molecule to the surface of the catalyst are no longer there. So B can drift off, and the catalyst is ready to do it again.
Most people sit very close to a heterogeneous catalyst every day — the catalytic converter in an automobile. It contains finely divided platinum and/or palladium metal and speeds up the reaction that causes harmful gases from gasoline (such as carbon monoxide and unburned hydrocarbons) to decompose into mostly harmless products (such as water and carbon dioxide).
Homogeneous catalysis: Offering an easier path
The second type of catalyst is a homogeneous catalyst — one that’s in the same phase as the reactants. It provides an alternative mechanism, or reaction pathway, that has a lower activation energy than the original reaction.
For an example, check out the decomposition reaction of hydrogen peroxide:
![]()
This is a slow reaction, especially if you keep the hydrogen peroxide cool in a dark bottle. The hydrogen peroxide in that bottle in your medicine cabinet may take years to decompose. But if you put a little bit of a solution containing the ferric ion in the bottle, the reaction will be much faster, even though it’ll be a two-step mechanism instead of a one-step mechanism:
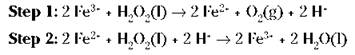
If you add the two preceding reactions together and cancel the species that are identical on both sides, you get the original, uncatalyzed reaction (species to be cancelled are bolded):
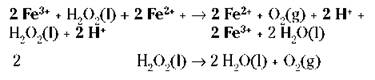
The ferric ion catalyst was changed in the first step and then changed back in the second step. This two-step catalyzed pathway has a lower activation energy and is faster.