Chemistry Essentials for Dummies
Chapter 9. Measuring Substances with the Mole
Chemical Reactions and Motes
When you’re working with chemical reactions, moles can help you figure out how much of a product you can expect to get based on how much of the reactants you have.
Take a look at my favorite reaction, the Haber process, which is a method of preparing ammonia (NH3) by reacting nitrogen gas with hydrogen gas:
![]()
In Chapter 7, I use this reaction over and over again for various examples (like I said, it’s my favorite reaction) and explain that you can read the reaction like this: one molecule of nitrogen gas reacts with three molecules of hydrogen gas to yield two molecules of ammonia:
![]()
If you like, you can scale everything up by a factor of 12:
12 molecules + 3(12 molecules) ↔ 2(12 molecules)
1 dozen molecules + 3 dozen molecules ↔ 2 dozen molecules
Or how about a factor of 6.022 x 1023? But wait a minute! Isn’t 6.022 x 1023 a mole? So you can write the equation like this:
![]()
6.022 x 1023 molecules + 3(6.022 x 1023) molecules ↔ 2(6.022 x 1023 molecules)
1 mole + 3 moles ↔ 2 moles
That’s right — not only can those coefficients in the balanced chemical equation represent atoms and molecules, but they can also represent the number of moles.
TIP. If you know the formula weight of the reactants and product, you can calculate how much you need and how much you’ll get. All you need to do is figure the molecular weights of each reactant and product and then incorporate the weights into the equation. Use the periodic table to find the weights of the atoms and the compound (see the earlier section “Counting by Weighing” for directions) and multiply those numbers by the number of moles, like this:
1 mol(28.014 g/mol) + 3 mol(2.016 g/mol) = 2 mol(17.031 g/mol)
28.014 g of N2 + 6.048 g of H2 = 34.062 g of NH3
Reaction stoichiometry
When you understand the weight relationships in a chemical reaction, you can do some stoichiometry problems. Stoichiometry refers to the mass relationship in chemical equations.
TIP. When you get ready to work stoichiometry types of problems, you must start with a balanced chemical equation. If you don’t have it to start with, go ahead and balance the equation.
Look at my favorite reaction — you guessed it — the Haber process:
![]()
Suppose that you want to know how many grams of ammonia can be produced from the reaction of 75.00 grams of nitrogen with excess hydrogen. The mole concept is the key. The coefficients in the balanced equation are not only the number of individual atoms or molecules but also the number of moles:
![]()
1 mole + 3 moles ↔ 2 moles
1 mol(28.014 g/mol) + 3 mol(2.016 g/mol) = 2 mol(17.031 g/mol)
First, convert the 75.00 grams of nitrogen to moles of nitrogen. Then use the ratio of the moles of ammonia to the moles of nitrogen from the balanced equation to convert to moles of ammonia. Finally, take the moles of ammonia and convert that number to grams. The equation looks like this:
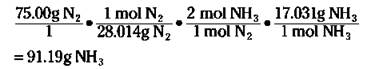
REMEMBER. A stoichiometric ratio — such as mol NH3/mol N2 — enables you to convert from the moles of one substance in a balanced chemical equation to the moles of another substance.
Percent yield
REMEMBER. In almost any reaction, you’re going to produce less of the product than you expected. You may produce less because most reactions are equilibrium reactions (see Chapter 8), because of sloppy technique or impure reactants, or because some other conditions come into play. Chemists can get an idea of the efficiency of a reaction by calculating the percent yield for the reaction using this equation:
![]()
The actual yield is how much of the product you get when you carry out the reaction. The theoretical yield is how much of the product you calculate you’ll get. The ratio of these two yields gives you an idea about how efficient the reaction is.
For the reaction of rust to iron (see the preceding section), your theoretical yield is 699.5 grams of iron; suppose your actual yield is 525.0 grams. Therefore, the percent yield is
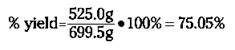
A percent yield of about 75 percent isn’t too bad, but chemists and chemical engineers would rather see 90+ percent. One industrial plant using the Haber reaction has a percent yield of better than 99 percent. Now that’s efficiency!
Limiting reactants
In a chemical reaction, you normally run out of one of the reactants and have some others left over. The reactant you run out of first is called the limiting reactant, and it determines the amount of product formed. (In some of the problems sprinkled throughout this chapter, I tell you which reactant is the limiting one by saying you have an excess of the other reactant(s).)
In this section, I show you how to calculate which reactant is the limiting reactant.
Here is a reaction between ammonia and oxygen.
![]()
Suppose that you start out with 100.0 grams each of both ammonia and oxygen, and you want to know how many grams of NO (nitrogen monoxide, sometimes called nitric oxide) you can produce. You must determine the limiting reactant and base your stoichiometric calculations on it.
To figure out which reactant is the limiting reactant, you calculate the mole-to-coefficient ratio: Calculate the number of moles of both ammonia and oxygen, and then divide each by their coefficients in the balanced chemical equation. The one with the smallest mole-to-coefficient ratio is the limiting reactant. For the reaction of ammonia to nitric oxide, you can calculate the mole-to-coefficient ratio for the ammonia and oxygen like this:
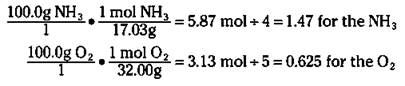
Ammonia has a mole-to-coefficient ratio of 1.47, and oxygen has a ratio of 0.625. Because oxygen has the lowest ratio, oxygen is the limiting reactant, and you need to base your calculations on it.
![]()
That 75.02 grams NO is your theoretical yield. But you can even calculate the amount of ammonia left over. You can figure the amount of ammonia consumed with this equation:
![]()
You started with 100.0 grams of ammonia, and you used 42.58 grams of it. The difference (100 grams - 42.58 grams = 57.42 grams) is the amount of ammonia left over.