Chemistry Essentials for Dummies
Chapter 2. What's In an Atom?
Locating Those Electrons
Many of the important topics in chemistry, such as chemical bonding, the shape of molecules, and so on, are based on where the electrons in an atom are located. Simply saying that the electrons are located outside the nucleus isn’t good enough; chemists need to have a much better idea of their location, so this section helps you figure out where you can find those pesky electrons.
The quantum mechanical model
Early models of the atom had electrons going around the nucleus in a random fashion. But as scientists discovered more about the atom, they found that this representation probably wasn’t accurate. Today, scientists use the quantum mechanical model, a highly mathematical model, to represent the structure of the atom.
This model is based on quantum theory, which says that matter also has properties associated with waves. According to quantum theory, it’s impossible to know an electron’s exact position and momentum (speed and direction, multiplied by mass) at the same time. This is known as the uncertainty principle. So scientists had to develop the concept of orbitals (sometimes called electron clouds), volumes of space in which an electron is likely present. In other words, certainty was replaced with probability.
The quantum mechanical model of the atom uses complex shapes of orbitals. Without resorting to a lot of math (you’re welcome), this section shows you some aspects of this newest model of the atom.
Scientists introduced four numbers, called quantum numbers, to describe the characteristics of electrons and their orbitals. You’ll notice that they were named by top-rate techno-geeks:
✓ Principal quantum number n
✓ Angular momentum quantum number l
✓ Magnetic quantum number ml
✓ Spin quantum number ms
Table 2-2 summarizes the four quantum numbers. When they’re all put together, theoretical chemists have a pretty good description of the characteristics of a particular electron.
Table 2-2. Summary of the Quantum Numbers
|
Name |
Symbol |
Description |
Allowed Values |
|
Principal |
n |
Orbital energy |
Positive integers (1,2, 3, and so on) |
|
Angular momentum |
l |
Orbital shape |
Integers from 0 to n - 1 |
|
Magnetic |
ml |
Orientation |
Integers from -l to +l |
|
Spin |
ms |
Electron spin |
+1/2 or -1/2 |
The principal quantum number n
The principal quantum number n describes the average distance of the orbital from the nucleus — and the energy of the electron in an atom. It can have only positive integer (whole- number) values: 1, 2, 3, 4, and so on. The larger the value of n, the higher the energy and the larger the orbital, or electron shell.
The angular momentum quantum number l
The angular momentum quantum number l describes the shape of the orbital, and the shape is limited by the principal quantum number n: The angular momentum quantum number l can have positive integer values from 0 to n - 1. For example, if the n value is 3, three values are allowed for l: 0, 1, and 2.
REMEMBER. The value of l defines the shape of the orbital, and the value of n defines the size.
Orbitals that have the same value of n but different values of l are called subshells. These subshells are given different letters to help chemists distinguish them from each other. Table 2-3 shows the letters corresponding to the different values of l.
Table 2-3. Letter Designation of the Subshells
|
Value of l (Subshell) |
Letter |
|
0 |
s |
|
1 |
p |
|
2 |
d |
|
3 |
f |
|
4 |
g |
When chemists describe one particular subshell in an atom, they can use both the n value and the subshell letter — 2p, 3d, and so on. Normally, a subshell value of 4 is the largest needed to describe a particular subshell. If chemists ever need a larger value, they can create subshell numbers and letters.
Figure 2-3 shows the shapes of the s, p, and d orbitals. In Figure 2-3a, there are two s orbitals — one for energy level 1 (1s) and the other for energy level 2 (2s). S orbitals are spherical with the nucleus at the center. Notice that the 2s orbital is larger in diameter than the 1s orbital. In large atoms, the 1s orbital is nestled inside the 2s, just like the 2p is nestled inside the 3p.
Figure 2-3b shows the shapes of the p orbitals, and Figure 2-3c shows the shapes of the d orbitals. Notice that the shapes get progressively more complex.
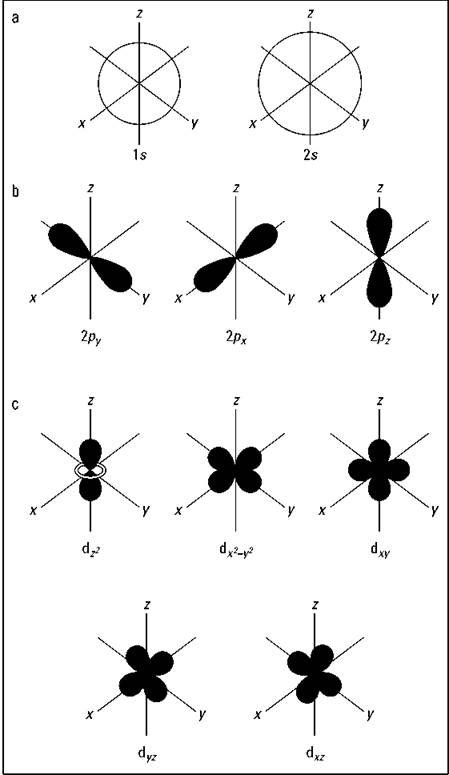
Figure 2-3: Shapes of the s, p, and d orbitals.
The magnetic quantum number ml
The magnetic quantum number ml describes how the various orbitals are oriented in space. The value of ml depends on the value of l. The values allowed are integers from -l to 0 to +l. For example, if the value of l = 1 (p orbital — see Table 3-4), you can write three values for ml: -1, 0, and +1. This means that there are three different p subshells for a particular orbital. The subshells have the same energy but different orientations in space.
Figure 2-3b shows how the p orbitals are oriented in space. Notice that the three p orbitals correspond to ml values of -1, 0, and +1, oriented along the x, y, and z axes.
The spin quantum number ms
The fourth and final quantum number is the spin quantum number ms. This one describes the direction the electron is spinning in a magnetic field — either clockwise or counterclockwise. Only two values are allowed for ms: +1/2 or -1/2 For each subshell, there can be only two electrons, one with a spin of +1/2 and another with a spin of -1/2.
Putting the quantum numbers together
Table 2-4 summarizes the quantum numbers available for the first two energy levels.
Table 2-4. Quantum Numbers for the First Two Energy Levels
|
n |
l |
Subshell Notation |
ml |
ms |
|
1 |
0 |
1s |
0 |
+1/2, -1/2 |
|
2 |
0 |
2s |
0 |
+1/2, -1/2 |
|
|
1 |
2p |
-1 |
+1/2, -1/2 |
|
|
|
|
0 |
+1/2, -1/2 |
|
|
|
|
+1 |
+1/2, -1/2 |
Table 2-4 shows that in energy level 1 (n = 1), there’s only an s orbital. There’s no p orbital because an l value of 1 (p orbital) is not allowed. And notice that there can be only two electrons in that 1s orbital (ms of +1/2 and -1/2). In fact, there can be only two electrons in any s orbital, whether it’s 1s or 5s.
Each time you move higher in a major energy level, you add another orbital type. So when you move from energy level 1 to energy level 2 (n = 2), there can be both s and p orbitals. If you write out the quantum numbers for energy level 3, you see s, p, and d orbitals.
Notice also that there are three subshells (ml) for the 2p orbital (see Figure 2-3b) and that each holds a maximum of two electrons. The three 2p subshells can hold a maximum of six electrons.
There’s an energy difference in the major energy levels (energy level 2 is higher in energy than energy level 1), but there’s also a difference in the energies of the different orbitals within an energy level. At energy level 2, both s and p orbitals are present. But the 2s is lower in energy than the 2p. The three subshells of the 2p orbital have the same energy. Likewise, the five subshells of the d orbitals (see Figure 2-3c) have the same energy.
Energy level diagrams
Chemists find quantum numbers useful when they’re looking at chemical reactions and bonding (and those are things many chemists like to study). But they find two other representations for electrons — energy level diagrams and electron configurations — more useful and easier to work with.
Chemists use both of these things to represent which energy level, subshell, and orbital are occupied by electrons in any particular atom. Chemists use this information to predict what type of bonding will occur with a particular element and to show exactly which electrons are being used. These representations are also useful in showing why certain elements behave in similar ways.
In this section, I show you how to use an energy level diagram and write electron configurations. I also discuss valence electrons, which are key in chemical reactions.
The dreaded energy level diagram
Figure 2-4 is a blank energy level diagram you can use to depict electrons for any particular atom. It doesn’t show all the known orbitals and subshells, but with this diagram, you should be able to do most anything you need to.
I represent orbitals with dashes in which you can place a maximum of two electrons. The 1s orbital is closest to the nucleus, and it has the lowest energy. It’s also the only orbital in energy level 1 (refer to Table 2-4). At energy level 2, there are both s and p orbitals, with the 2s having lower energy than the 2p. The three 2p subshells are represented by three dashes of the same energy. The figure also shows energy levels 3, 4, and 5.
TIP. Notice that the 4s orbital has lower energy than the 3d: This is an exception to what you may have thought, but it’s what’s observed in nature. Go figure.
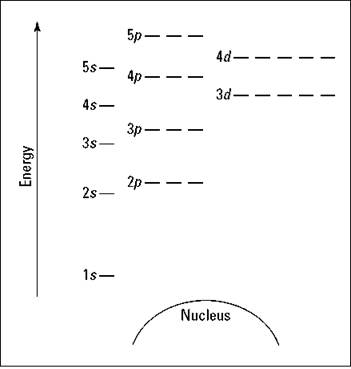
Figure 2-4: An energy level diagram.
Speaking of which, Figure 2-5 shows the Aufbau principle, a method for remembering the order in which orbitals fill the vacant energy levels.
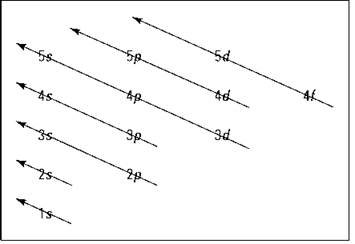
Figure 2-5: The Aufbau filling chart.
REMEMBER. In using the energy level diagram, remember two things:
✓ Electrons fill the lowest vacant energy levels first.
✓ When there’s more than one subshell at a particular energy level, such as at the 3p or 4d levels (see Figure 2-4), only one electron fills each subshell until each subshell has one electron. Then electrons start pairing up in each subshell. This rule is named Hund’s rule.
Suppose you want to draw the energy level diagram of oxygen. You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has eight protons in its nucleus and eight electrons. So you put eight electrons into your energy level diagram. You can represent electrons as arrows, as in Figure 2-6. Note that if two electrons end up in the same orbital, one arrow faces up and the other faces down. This is called spin pairing. It corresponds to the +1/2 and -1/2 of ms (see “The spin quantum number ms” section, earlier in this chapter, for details).
The first electron goes into the Is orbital, filling the lowest energy level first, and the second one spin-pairs with the first one. Electrons 3 and 4 spin-pair in the next-lowest vacant orbital — the 2s. Electron 5 goes into one of the 2p subshells (no, it doesn’t matter which one — they all have the same energy), and electrons 6 and 7 go into the other two totally vacant 2p orbitals. The last electron spin-pairs with one of the electrons in the 2p subshells (again, it doesn’t matter which one you pair it with). Figure 2-6 shows the completed energy level diagram for oxygen.
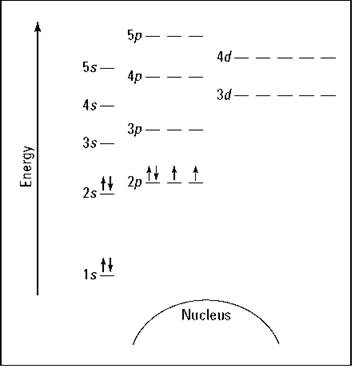
Figure 2-6: Energy level diagram for oxygen.
Electron configurations
Energy level diagrams are useful when you need to figure out chemical reactions and bonding, but they’re very bulky to work with. Wouldn’t it be nice if there were another representation that gives just about the same information but in a much more concise form? Well, there is. It’s called the electron configuration.
The electron configuration for oxygen is 1s22s22p4. Compare that notation with the energy level diagram for oxygen in Figure 2-6. Doesn’t the electron configuration take up a lot less space? You can derive the electron configuration from the energy level diagram. The first two electrons in oxygen fill the 1s orbital, so you show it as 1s2 in the electron configuration. The 1 is the energy level, the s represents the type of orbital, and the superscript 2 represents the number of electrons in that orbital. The next two electrons are in the 2s orbital, so you write 2s2. And finally, you show the four electrons in the 2p orbital as 2p4. Put it all together, and you get 1s22s22p4.
TIP. The sum of the superscript numbers equals the atomic number, or the number of electrons in the atom.
Here are a couple of electron configurations you can use to check your conversions from energy level diagrams:
Chlorine (Cl): 1s22s22p63s23p5
Iron (Fe): 1s22s22p63s23p64s23d6
Valence electrons: Clues about chemical reactions
Knowing the number of electrons that are an atom’s outermost energy level gives you a big clue about how that atom will react.
When chemists study chemical reactions, they study the transfer or sharing of electrons. The electrons more loosely held by the nucleus — the electrons in the energy level farthest away from the nucleus — are the ones that are gained, lost, or shared.
REMEMBER. Electrons are negatively charged, and the nucleus has a positive charge due to the protons. The protons attract and hold the electrons, but the farther away the electrons are, the less the attractive force.
The electrons in the outermost energy level are commonly called valence electrons. Chemists really only consider the electrons in the s and p orbitals in the energy level that’s currently being filled as valence electrons. In the electron configuration for oxygen, 1s22s22p4, energy level 1 is filled, and there are two electrons in the 2s orbital and four electrons in the 2p orbital for a total of six valence electrons. Those valence electrons are the ones lost, gained, or shared.