The Handy Chemistry Answer Book (2014)
INORGANIC CHEMISTRY

STRUCTURE AND BONDING
Which molecules are considered inorganic?
Any molecule without a carbon atom is technically considered inorganic, but in practice, there are some exceptions. Many salts (like carbonates, CO32−, or cyanides, CN−) are thought of as inorganic, even though they do contain carbon.
What elements are metals?
Metals are elements that easily conduct electricity. There are a number of sets of metals in the periodic table. Instead of just listing the metallic elements, let’s take a look at those sets one at a time in the next few questions.
What are the alkali metals and alkaline earth metals?
The first two columns of the periodic table are known as the alkali (Group 1) and alkaline earth (Group 2) metals. The elements in both groups have very low ionization energies, which means they readily give up one or two electrons to reach a Noble gas electron configuration (e.g., Na+, Mg2+). In their elemental forms, these elements are soft, silver-colored substances. These elements are almost never found in their pure elemental state in nature because they quickly react with air or moisture, sometimes violently.
What are the transition metals?
Elements in the d-block (Groups 3–12) of the periodic table are referred to as transition metals. (The lanthanides and actinides are usually excluded.) With the exception of Group 12, the transition metal elements have an incomplete d-shell of electrons. The chemistry of these elements depends strongly on these d-electrons, and most transition metals are stable in multiple different oxidation states. If the metal has any unpaired d-electrons, it can have magnetic properties. Many transition metals have uses as catalysts in bond-forming reactions, and we’ll talk about a few later in this chapter.
What is a metalloid?
Metalloid is a term for elements that are sort-of metals, and sort-of not metals. Sometimes this group of elements is referred to as semimetals. To be more precise, these elements exhibit some of the physical and chemical properties of metals. Generally metalloids have some electrical conductivity, but not nearly as much as true metals. Because of these ambiguous definitions, even which elements are called metalloids can vary. Usually boron, silicon, germanium, arsenic, antimony, and tellurium are included as metalloids; sometimes polonium and astatine; rarely selenium.
What are the valence orbitals of transition metals?
The transition metals are also referred to as the d block because their valence orbitals are the d shell (see illustration on this page). There are five different d orbitals. Three (dxy, dxz, and dyz) of these five look similar, just with different orientations in space. The fourth (dx2-y2) has the same shape as the previous three, but the loops point in along the axes as opposed to between them. Finally the dz2 looks like a p-orbital pointed along the z-axis with a ring (technically a torus) around it.
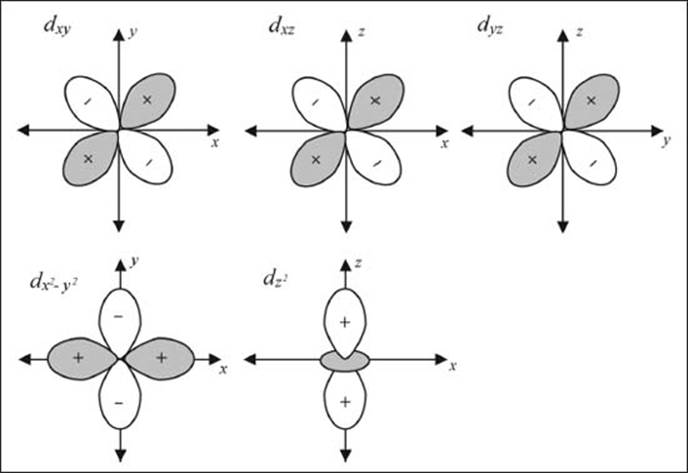
The five different valence orbitals of transition metals.
What are the lanthanides and actinides?
The actinides and lanthanides make up the f-block of the periodic table. These two rows are frequently separated from the main table, but that’s just so the periodic table isn’t so wide on a piece of paper (seriously). Many of these elements have radioactive isotopes, but the rate at which they decay can vary immensely. For example, 238U has a half-life of 4.5 billion years, but 234Pa has a half-life of only seventy-two seconds!
What is crystallography?
Crystallography is the study of the arrangement of the atoms in a solid material. Today, this term generally refers to methods that rely on the patterns of photons (commonly, X-rays), neutrons, or electrons that are diffracted after impacting a sample. The patterns of the diffracted radiation or particles can be interpreted to determine the structure inside the crystal. The interpretation of the diffraction patterns to yield a chemical structure is by no means a simple task, but crystallographers have been doing this for a long time and it has become a commonplace technique. Crystallographic methods have been used for decades to study the structures of inorganic solids and organometallic complexes.
We point out that, while crystallographic methods are commonly used to study inorganic compounds, they have also frequently been applied for studying other types of molecules as well, including biomolecules. While it can often be difficult to obtain a crystalline sample of a biomolecule, such as a protein, crystallography can be extremely useful in deducing protein structures.
What is a crystal lattice?
Crystalline solids have regular, repeating arrangements of atoms or molecules. In order to classify these arrangements, chemists use a three-dimensional lattice to encompass the smallest repeat unit (known as the unit cell) of the crystal structure. There is really a lot of math involved, but we can explain it visually. A unit cell is really just a tiny box containing some atoms or molecules. We draw the edges of this small box so that if you were to line up lots of identical copies of this box in all directions, you would get the structure of the entire crystal lattice.
Are all crystal lattices cubic?
In other words, are all the three axes of the unit cell always the same length? No. In fact, cubic is only one of the seven “crystal systems.” These systems are based on the lengths and angles of the unit cell. The cubic group has three sides of equal length, and all the internal angles are 90°. If one side is longer, we get a tetragonal lattice. If all three sides are different lengths, the lattice system is called orthorhombic. If one angle is not 90°, it’s monoclinic. If the angles are all equal but they’re not 90°, it’s a rhombohedral lattice. If the angles are all different and not 90°, the lattice is triclinic.
Yes, you’re right, that’s only six. The seventh isn’t based on a cube at all. The hexagonal lattice system is (you guessed it) based on a hexagon.
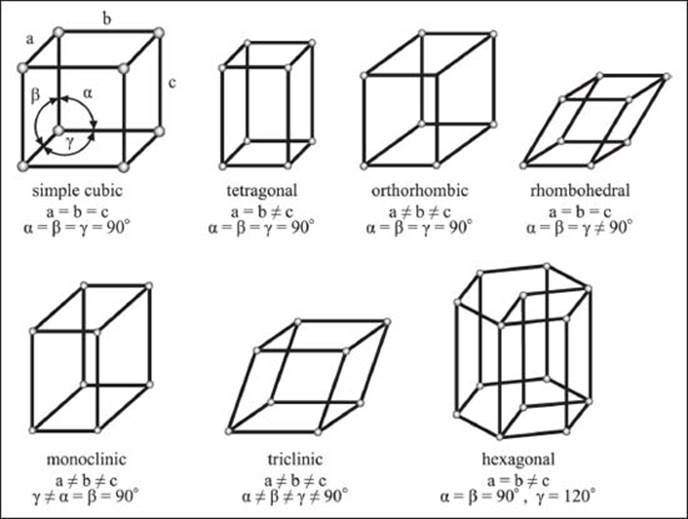
The seven crystal systems.
What packing arrangements are possible for a crystal lattice?
There are three basic packing arrangements, which we can describe by again imagining a tiny box. If we place an atom at each of the eight vertices of this box, the arrangement is referred to as simple cubic. If we take a simple cubic unit cell and add an atom to the center of each face, we have a face-centered cubic arrangement. If we instead add an atom to the center of the cube, it’s called a body-centered cubic unit cell. There are more possibilities, but these three simple ones described here cover a lot of the crystals that chemists encounter.
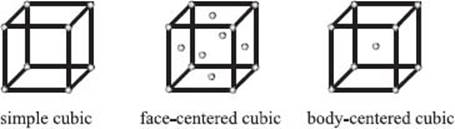
What determines the most favorable packing arrangement?
Thermodynamics! Okay, that’s a cop-out answer. Attractive interactions (like Van der Waals forces or hydrogen bonding) can play a large role in determining the stability of a crystal lattice. The most stable packing arrangement can also depend on the pressure and temperature at which the crystal forms.
Can more than one packing arrangement exist for a given chemical molecule?
Yes—this is referred to as polymorphism, and examples are known for most types of crystalline materials—organic and inorganic molecules, polymers, and metals. The way in which molecules pack in the solid state can actually alter some of its properties. Some pharmaceutically active molecules have more than one solid-state structure, or polymorphs. Sometimes certain polymorphs of drugs can be more useful. For example, a specific arrangement could be more soluble, making it more active in the human body. A second polymorph of aspirin (acetylsalicylic acid) was discovered in 2005, but it’s only stable at -180 °C.