The Handy Chemistry Answer Book (2014)
INORGANIC CHEMISTRY
ORGANOMETALLIC CHEMISTRY
What is an organometallic complex?
These are molecules with bonds between carbon and a metal. This includes alkali and alkali earth metals (Groups 1 and 2 of the periodic table), the transition metals (Groups 3–12, including the f block), and sometimes Group 13 metals are also included. The bonds between metal and carbon can vary widely in their ionic or covalent character. The bonding between a metal and carbon is mostly ionic in two situations: (1) if the metal is very electropositive, as in Groups 1 and 2; or (2) if the carbon group is a stable anion (by being delocalized via resonance as in cyclopentadienyl anion). There are also organometallic molecules with more covalent bonds between the metal atom and the carbon atom. This is usually seen with the transition metals or elements like aluminum. The nature of the metal-carbon bond plays an important role in how organometallic complexes react. The resonance structures of the cyclopentadienyl anion are below:
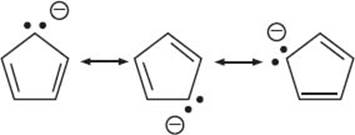
What is the 18-electron rule?
Earlier we talked about the octet rule, which says that elements like carbon and oxygen are most stable when they have eight valence electrons. For transition metals, as we’ve mentioned, the d-orbitals start to become important, so eight electrons just aren’t enough anymore. Since there are five d-orbitals, we need an additional ten electrons to fill the valence shell. And 8 + 10 = 18, hence the 18-electron rule for transition metals.
How does one determine the oxidation state of an organometallic complex?
There are a few ways to approach this problem (and, of course, every chemist thinks the way they use is the most correct), but let’s look at what is hopefully the simplest way to think about this. We’re going to only talk about complexes with metal-carbon bonds and no other types of ligands. If you’re curious enough to want to know how nitroxide binds a transition metal, chances are you have an inorganic chemistry textbook at home anyway.
Okay, let’s look at tetra(methyl) zirconium, which has no net charge. In this counting technique, all of the electrons in the metal-carbon bonds are placed on the carbon atoms. So we get four methyl anions and one zirconium ion. Since this complex has no net charge, the zirconium center must balance out the net four negative charges from the methyl groups. Zirconium must be in the +4 oxidation state. Easy, right?
One more example: K3[Fe(CN)6] or potassium hexacyanoferrate. We again move the electrons in the metal-carbon bonds to the carbon groups, making six cyanide ions (CN−), an iron ion, and three potassium ions. We have six negative charges (6 CN−) and three positive charges (3 K+ ions), so to balance out the charges, the iron center must be in the +3 oxidation state.
ZrMe4 4 Me1- + Zr4+
K3 [Fe(CN)6] 3 K1+ + 6 CN1- + Fe3+
It’s important to remember that when we count electrons in this way, we’re doing only that—counting. Don’t get the idea that all of these metal-carbon bonds are the same, or that they’re all ionic bonds—this is just for electron bookkeeping!
Why do organometallic complexes make good catalysts?
Catalysts, by definition, provide a lower-energy route to the product. Organometallic complexes react with organic molecules in ways that are very different than how organic molecules react with themselves. At the most basic level, this is because transition metals have d-orbitals that are involved in making and breaking chemical bonds. With additional orbitals that are of different symmetry than the s- and p-orbitals that are available to elements like carbon, nitrogen, and oxygen, organometallic complexes can accomplish feats that are just impossible (forbidden!) for other elements.
Are inorganic elements important in biological systems?
Absolutely! Not only do ions like sodium and potassium play a huge role in nervous systems, but many enzymes (nature’s catalysts) have metal ions in their active sites. Go find a bottle of vitamins and see just how many metals you need daily!
What is the hapticity of an organometallic ligand?
The hapticity of an organometallic ligand is a pretty simple concept—it just tells how many atoms from a given ligand are coordinated to the metal center. This is typically denoted in chemistry literature with the Greek letter “eta.” A η2 thus involves a ligand with two atoms coordinated to the metal center, a η1 one atom, and so on. The most common hapticity for a ligand is η1, but it is not uncommon to find ligands, such as the cyclopentadienyl anion ligand (usually η5), with higher coordination numbers.
What is a carbene ligand?
A carbene is a molecule that contains a carbon atom with two bonds and two unpaired valence electrons. This leaves the carbon atom neutral in terms of formal charge (see “Atoms and Molecules”), but still typically much more reactive than a typical carbon atom with four bonds. Carbenes are often found coordinated to metal centers in organometallic complexes. These carbene ligands are less reactive than a free carbene species, and actually you might be surprised to learn that organometallic carbene complexes aren’t always prepared from the reactions of free carbenes with metal centers. As an organometallic ligand, a carbene may be be either electrophilic or nucleophilic at the carbon atom (see “Organic Chemistry”). Carbene ligands that are electrophilic at carbon are termed Fischer carbenes, and those that are nucleophilic at carbon are called Schrock carbenes. A third class of particularly unreactive carbene ligands are termed persistent (or Arduengo) carbenes.
What is a monodentate ligand?
A monodentate ligand is a ligand that coordinates to a metal center via a single atom on the ligand.
What is a polydentate ligand?
A polydentate ligand is a ligand that coordinates to a metal center via two or more atoms, forming two or more bonds. This resulting complex is known as a chelate complex.
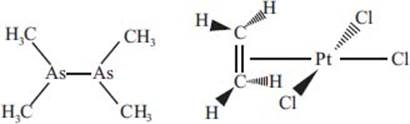
What are some of the first organometallic complexes, and when were they discovered?
Among the earliest organometallic complexes was a compound known as cacodyl, or tetramethyldiarsine. This compound, with the chemical formula C4H12As2 (shown below at left), was first discovered in 1760 and was known for its toxicity and terrible odor. The first platinum olefin complex, C2H4Cl3KPt (also known as Zeise’s salt; see below, right), was discovered in 1829. This early organometallic complex was influential in establishing some of the essential underlying concepts that have since become crucial to organometallic chemistry. These examples represent just a couple of the first organometallic species, and to date, thousands of organometallic complexes are known.
What is a carbon-hydrogen bond activation reaction?
Carbon-hydrogen activation reactions are very much what the name would imply— they break typically unreactive carbon-hydrogen bonds. Considering the prevalence of carbon-hydrogen bonds in organic compounds, this is another extremely important type of reaction to be able to carry out both effectively and with selectivity. Successful examples of carbon-hydrogen bond activation reactions have only come about relatively recently (with the first genuine example reported ca. 1965), and organometallic reagents have played a key role in the development of carbon-hydrogen bond reactions.
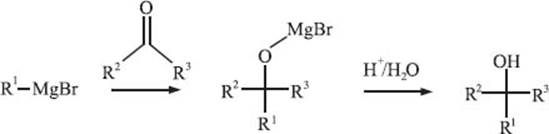
What is the Grignard reaction?
The Grignard reaction is one of the most well-known and powerful reactions in organo-metallic synthesis, primarily due to its ability to readily form new carbon-carbon bonds. In this reaction, a Grignard reagent reacts by adding to a carbonyl functional group of an aldehyde or ketone and forms a new carbon-carbon bond at the carbonyl carbon. Grignard reagents, which are organometallic species that carry out the Grignard reaction, are typically formed by adding magnesium metal to an alkyl or aryl halide (R1-Br in the scheme below). The discovery of this important reaction was awarded with a Nobel Prize in 1912, and the reaction is actually also named after the French chemist François Auguste Victor Grignard, who discovered it.
Do reactions always take place at the metal center of an organometallic complex?
Not always. Reactions can also take place at the ligands of a complex! For example, a nu-cleophile can add to an alkene ligand while it is coordinated to a metal center.
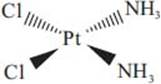
What is cisplatin, and how does it help to fight cancer?
Cisplatin is a platinum-based compound (see structure below) that reacts with DNA causing it to crosslink, eventually leading to programmed cell death, also known as apoptosis. Cisplatin was the first in a class of platinum containing anticancer drugs. It has been used to fight several types of cancer and is particularly effective against testicular cancer.
What is the valence shell electron pair repulsion model?
The valence shell electron pair repulsion model (VSEPR) is used to predict the bonding geometry about a central atom based on a set of rules that predicts the repulsive forces between the electrons in chemical bonds and in lone pairs. In this model, two factors are used to predict the bonding geometry: the steric number and the number of lone pairs of nonbonding electrons. The steric number is defined as the number of atoms bonded to the central atom plus the number of lone pairs of nonbonding electrons. Based on these two numbers, the table below predicts the bonding geometry that will be observed about a central atom. It should be noted that this is just a predictive model, and it will not be correct 100% of the time.
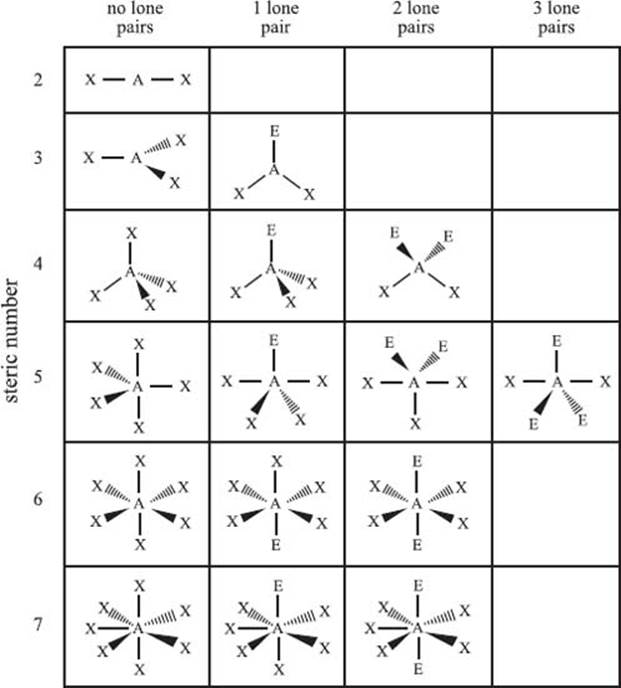
From top row down, and left to right, the types of bonding geometries are: Steric Number 2) linear; Steric Number 3) trigonal planar, bent; Steric Number 4) tetrahedral, trigonal pyramidal, bent; Steric Number 5) trigonal bipyramidal, seesaw, T-shaped, linear; Steric Number 6) octahedral, square pyramidal, square planar; Steric Number 7) pentagonal bipyramidal, pentagonal pyramidal, and pentagonal planar.
What is group theory and how is it useful in chemistry?
Group theory, in the chemistry sense, involves making use of the symmetry of a molecule to better understand its physical properties. By examining the symmetry properties of a molecule, it can be placed into a “point group” that describes the symmetry operations (rotations of reflections) that characterize the molecule’s symmetry. Group theory allows chemists to understand an impressive number of molecular properties including the spacing of energy levels of the orbitals in a molecule, the symmetry of the molecular orbitals, the types of transitions that can occur (like vibrational and electronic excitations), and what the delocalized vibrational motions of a molecule look like. It is very impressive that symmetry can tell us so much about molecules without the need for any complex calculations!
When doctors give people “lithium,” is that the same lithium as in lithium-ion batteries?
It’s the same element, yes. Lithium has quite a few interesting applications! It is used to treat bipolar disorder and in lithium and lithium-ion batteries. It is a metal that can be cut using a spoon, is used to make thermonuclear igniters, and also as a component of armor for army vehicles like tanks. All of this from the tiny element lithium.
What are “hard” and “soft” Lewis acids and bases?
The words “hard” and “soft” are commonly used to describe two broad classes of Lewis acids and bases (See “Chemical Reactions”). Hard acids and bases typically have small atomic (or ionic) radii, high oxidation states, high electronegativity (for bases), and are not very polarizable. Soft acids and bases tend to be the opposite in that they have relatively large atomic (or ionic) radii, low oxidation states, low electronegativity, and are highly polarizable. As it turns out, hard acids tend to react more rapidly and form stronger bonds with hard bases, and the same is true for soft acids paired with soft bases. This pattern is what makes the theory of hard and soft acids and bases useful for predicting and understanding reactivity in inorganic complexes.
What happens when you add sodium metal to water?
Sodium metal reacting with water can produce a violent response! Sodium and water react according to the equation:
2 Na (s) + 2 H2O (l) ![]() 2 NaOH (aq) + H2 (g) + heat
2 NaOH (aq) + H2 (g) + heat
As you can see, this reaction produces heat, and it turns out that the amount of heat produced can be pretty substantial. The heat can even cause the hydrogen gas (produced in the reaction) to ignite, thus reacting according to this equation:
H2 (g) + O2 (g) ![]() H2O (g) + heat
H2O (g) + heat
The heat produced by both of these reactions can even cause any currently unreacted sodium metal to also ignite and burn according to the equation:
Na (s) + O2 (g) ![]() Na2O2 (s)
Na2O2 (s)
From these reactions, it is probably clear that the reaction of sodium with water can produce quite a lot of heat!
What is electron paramagnetic resonance spectroscopy?
Electron paramagnetic resonance (EPR) spectroscopy, also known as electron spin resonance (ESR) spectroscopy, is a method used to probe unpaired electrons in a molecule. This method is fairly similar to nuclear magnetic resonance (NMR) (see “The Modern Chemical Lab”), but it involves exciting electronic spin states instead of nuclear ones. One downside to this technique, relative to NMR, is that the majority of molecules do not contain unpaired electrons and thus cannot be studied using EPR. On the other hand, the lack of interfering signals from most solvents and other molecules can very often be an advantage for the same reason.
How is inorganic chemistry relevant to biological chemistry?
Because metals are important to many biological processes! In the active sites of enzymes (See “Biochemistry”), where the important chemical reactions take place, metal centers are frequently crucial to the catalytic bond-forming and bond-breaking events. Metals are also important for maintaining gradients in ion concentrations, allowing muscles to move, and a variety of other biological processes. Bioinorganic chemistry is thus a huge field, and these two topics are often taught in conjunction with one another.
Why do metals make for such effective active sites in enzyme catalysis?
Metals are crucial components in enzymatic catalysis for largely the same reasons that organometallic complexes make good catalysts. These include the fact that many metal centers can complex to a variety of substrates, undergo facile changes in oxidation state, and provide good electron donors or acceptors. These characteristics can, and often do, work together to accomplish some remarkable chemical feats.
What are MRI contrast agents and what are they used for in medicine?
Magnetic resonance imaging (MRI) (see “The Modern Chemical Lab”) contrast agents are used to make tissues and other structures inside the body easier to view in an MRI scan. Many of these contrast agents are based on gadolinium with various ligands attached. The contrast agents are injected or ingested into the body, and they function by changing the relaxation time of the atoms observed in an MRI scan. The overall result is that these complexes improve the ability of MRI to see what is going on in your body.
How are transition metals important for nitrogen fixation?
Nitrogen fixation is the term for a process that converts diatomic nitrogen gas (N2) in the atmosphere into ammonia (NH3). This is a very important biological process because it converts N2, which is very unreactive, into a form that can more readily be incorporated into amino acids and other molecules. Transition metals (such as vanadium or molybdenum) are found in the active sites of the enzymes that carry out this important reaction.
Why is calcium important for strong bones?
Your bones need calcium to stay strong because they are made of a calcium-based substance called calcium hydroxylapatite, with the chemical formula Ca5(PO4)3(OH). It is recommended that people between the ages of 18–50 consume about 1000 milligrams of calcium per day, while older people should consume 1200–1500 milligrams of calcium each day.
How are metals important in maintaining osmotic balance in cells?
The metals sodium and potassium (really their ions, Na+ and K+) are responsible for moderating ion and concentration gradients across cellular membranes. These ions can be selectively allowed to pass, or be pumped, across cellular membranes.
How are transition metals important in photosynthesis?
Chlorophyll, the green pigment that plays a crucial role in photosynthesis, contains an atom of magnesium at the center of its porphyrin ring (a porphyrin ring is an organic ring molecule found in many biochemical systems). The Mg atom plays a crucial role in the absorption of light from the Sun, the energy from which is then channeled through the molecule and is eventually put to work by the plant’s cells.
What metals are naturally present in biological systems?
Sodium, magnesium, vanadium, chromium, manganese, iron, copper, nickel, cobalt, zinc, molybdenum, and tungsten are all naturally present in varying quantities in biological systems.
What metals are used as probe agents in biological systems?
Yttrium, technetium, gold, silver, platinum, mercury, and gadolinium are used as probe agents in biological systems.
What metal is at work in alcohol dehydrogenase in your liver?
The active site of alcohol dehydrogenase contains a zinc center that is responsible for catalyzing the reaction of ethanol to acetaldehyde.