The Handy Chemistry Answer Book (2014)
ANALYTICAL CHEMISTRY
ELECTROCHEMISTRY
What is electrochemistry?
Electrochemistry is a branch of chemistry that deals with electrons transferring between an interface and a molecule in an electrolyte solution. The interface is typically a conducting metallic material. Electrochemistry is a rich field that has led to the development of batteries, a widely used chemical separation technique called electrophoresis, a process for plating metals known as electroplating, and an immense body of knowledge surrounding oxidation-reduction chemistry, among other achievements.
What is an electrochemical cell?
One common example of a redox reaction in electrochemistry involves the transfer of electrons from zinc (Zn) metal to copper (Cu) ions in an electrochemical cell.
Zinc (Zn) gives up electrons more readily than copper (Cu), so electrons spontaneously transfer from the Zn metal to the Cu metal, depositing Zn2+ ions into the solution and causing Cu metal to come out of the solution and onto the solid. This process releases energy, which can be used to drive external processes (such as powering a lightbulb, for example).
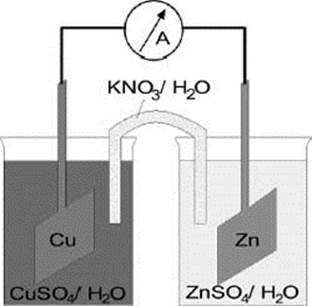
In this example of an electrochemical cell, a piece of zinc (Zn) and a piece of copper (Cu) are placed in solutions of zinc sulfate and copper sulfate, respectively, connected by a salt bridge of potassium nitrate. Electrons are given up by the zinc anode and transferred to the copper cathode, generating an electrical current.
How can metals be plated onto a surface?
Metals can be plated onto surfaces using a technique from electrochemistry called electroplating. Positively charged metal ions dissolved in a solution are attracted to a negatively charged electrode. This electrode reduces (gives electrons to) the positively charged metal ions, making them into a neutral metal species. The neutral metal is no longer soluble, so it forms a coating on the surface of the electrode.

Rechargable batteries work because the chemical reactions that occur inside them can be more easily reversed than in non-rechargable batteries.
How does a battery work?
Batteries operate based on chemical redox reactions that are set up to cause the spontaneous flow of electrons through the object you want to power.
Why can some batteries be recharged and others cannot?
Batteries produce electricity from a chemical reaction. So to recharge a battery, we would have to be able to reverse the chemical reaction by applying an electric current. In truth most batteries use chemical reactions that could be reversed, but there are efficiency considerations that come into play. Rechargeable batteries make use of reactions that can be reversed easily, many times, without too much degradation of the battery materials. One-time-use batteries typically use reactions that could only be reversed and reused a couple of times before they would not work very well anymore.
What is the reduction potential of an electrochemical cell?
The reduction potential of a species describes its tendency to accept electrons, or, in other words, to be reduced. A full electrochemical cell typically involves two separate half-reactions, each of which is associated with its own reduction potential. The overall reduction potential for an electrochemical cell is the difference of the reduction potentials for the two half-reactions.
What is the Nernst equation?
The Nernst equation relates the reduction potential of an electrochemical cell to a standard-state reduction potential, along with the temperature, reaction quotient, and number of electrons transferred during the reaction in question. It was first developed by Walther Nernst, who won the Nobel Prize in Chemistry in 1920 for his influential work in physical chemistry.
What is calorimetry and what is it used for?
Calorimetry involves measuring the amount of energy released during a chemical reaction in the form of heat. This is accomplished using a device called a calorimeter. A calorimeter is a container inside which a chemical reaction can be carried out while thermally insulated from the surroundings. This can even be something as simple as a sealed Styrofoam® coffee cup with a thermometer inserted through the lid (though more sophisticated devices certainly do exist). The temperature change inside the calorimeter can be monitored to determine the amount of energy given off during the chemical reaction, which can then be used to determine the enthalpy change associated with the reaction.
What is flame ionization detection?
Flame ionization detection (FID) is a method of detecting organic analytes in gas-phase chromatography experiments. A very hot flame is used to burn the analytes emerging from the chromatography instrument, which produces positively charged ions. These ions are then attracted to a negatively charged electrode, which detects them by generating an amount of current proportional to the number of positively charged carbon atoms reaching the electrode. FID can be particularly useful due to the fact that it detects organic analytes without any interference from a wide variety of other gases, which can be present in the sample or in the carrier gas used in the gas chromatograph. There are some downsides to FID, one of which is that the sample is destroyed as it is analyzed.