The Handy Chemistry Answer Book (2014)
PHYSICAL AND THEORETICAL CHEMISTRY
FASTER THAN A SPEEDING WAVE
How fast does light travel?
In a vacuum, the speed of light is about 3 × 108 meters per second, which is very, very fast. That’s so fast that a beam of light could travel around the whole world in only about 0.13 seconds!
It’s interesting to consider just how far away from the Earth stars really are. After the Sun, the next closest star to the Earth is over four light years away (a light year is the distance light travels in one year). This means the next closest star is over 20,000,000,000,000 miles away. Because light must reach our eyes for us to see anything, if that star exploded, we wouldn’t see it until over four years after it actually happened!
Can anything travel faster than the speed of light in a vacuum?
No, or at least it’s presently thought that this would be impossible. It’s interesting, though, that there have been a couple of experiments in recent years which have observed particles called neutrinos moving faster than the speed of light. Even the scientists who carried out these experiments questioned the results, however, and they encouraged others to try to confirm their results or to find where a mistake might have been made in their measurements. In the end, they did find out that these results were caused by a mistake; there was a loose electrical cable that caused enough error to invalidate the results.
Is the speed of light always the same?
The speed of light actually depends on what material the light is moving through! Each material has a property called an index of refraction. From the index of refraction, we can calculate the speed of light passing through a material from the following equation:
ν = c/n
In this equation, c is the speed of light in a vacuum (about 2.998 × 108 m/s), n is the index of refraction of the material in question, and ν tells us the velocity of light in the material.
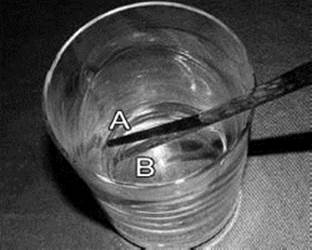
This photo combines two images: one of a stick partly submersed in a glass of water (A), and the same picture without water in the glass (B). The stick seems bent in A because light is refracted as it leaves the water and is perceived by your eyes.
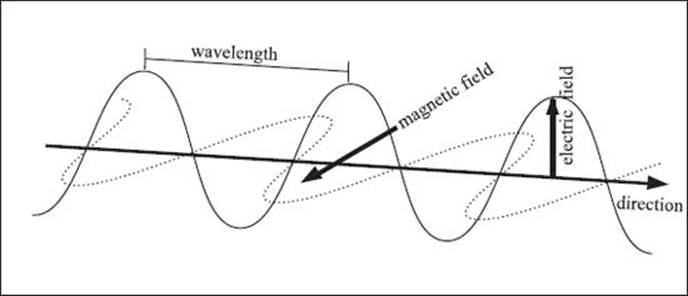
Electromagnetic radiation consists of electric and magnetic fields that run perpendicular to one another. The wavelength of light is the distance measured between wave crests.
What are the wavelength and frequency of light?
The light we see is a form of energy called electromagnetic radiation. It may sound like something complicated, but it’s nothing too exotic or unfamiliar. Everything you see is because of electromagnetic radiation. Electromagnetic radiation consists of perpendicular electric and magnetic fields that oscillate in amplitude. The number of times per second that the fields oscillate is the frequency of the radiation. This is measured in Hertz (Hz), or inverse seconds. The wavelength is the distance that the light travels through space during one oscillation of the electric or magnetic field.
What is the electromagnetic spectrum?
The electromagnetic spectrum describes the entire range of frequencies (or wavelengths) possible for electromagnetic radiation to have. In principle, the spectrum is practically infinite, though there are limitations on how high or low of frequencies we can practically achieve and work with. On the high end of the frequency spectrum are usually gamma rays, with frequencies of around 1020 Hz, while on the low end are “extremely low frequencies” of only a few Hz.
Electromagnetic Spectrum
|
Type |
Frequency (Hz) |
Wavelength (cm) |
|
Radio |
< 3 × 1011 |
> 10 |
|
Microwave |
3 × 1011 – 1013 |
10 – 0.01 |
|
Infrared |
1013 – 4 × 1014 |
0.01 – 7 × 10−5 |
|
Visible |
4 – 7.5 × 1014 |
7 × 10−5 – 4 × 10−5 |
|
Ultraviolet |
1015 – 1017 |
4 × 10−5 – 10−7 |
|
X-rays |
1017 – 1020 |
10−7 –10−9 |
|
Gamma Rays |
1020 – 1024 |
< 10−9 |
How is the frequency of electromagnetic radiation related to its energy?
The frequency of a photon is related to its energy, E, by a pretty straightforward equation:
E = h
In this equation, h is Planck’s constant, which has a value of 6.626 × 10−34 J•s. The frequency term, , is the frequency of the radiation in Hz. As we can see from this equation, electromagnetic radiation with higher frequency has higher energy.