The Handy Chemistry Answer Book (2014)
PHYSICAL AND THEORETICAL CHEMISTRY
BIG FREAKING LASERS
What is spectroscopy?
Spectroscopy is a branch of science associated with using light to study transitions between energy levels. Not all scientists who use spectroscopy (spectroscopists) are physical chemists, though physical chemists (and physicists) are typically the people who develop new spectroscopic methods and experimentally investigate the details of how light interacts with matter. Data collected in spectroscopic experiments is typically presented as some response of an atomic or molecular system as a function of frequency/wavelength or time. When the response is plotted as a function of frequency/wavelength, it is called a spectrum.
What are Fraunhofer lines?
When scientists first began observing the spectrum of light reaching Earth from the Sun, the spectrum contained many dark lines, which indicated that light of certain wavelengths wasn’t present in the sunlight. These are now called Fraunhofer lines (named after their discoverer), and they are caused by the elements in the outer atmosphere of the Sun absorbing certain wavelengths of light, which prevents those wavelengths from reaching the Earth. Understanding that Fraunhofer lines were caused by atomic absorptions was one of the earliest examples of atomic spectroscopy.
What are ground and excited electronic states?
The ground electronic state of an atom or molecule is the lowest energy electronic state. Excited states are any electronic states that have a higher energy than the lowest energy electronic state.
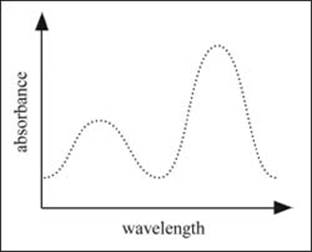
An example of a typical spectrum plotting absorbance versus wavelength.
How can light cause transitions between energy levels?
Light comes in discrete units called photons, and each photon has a particular energy associated with it. When a photon’s energy matches the energy spacing between two energy levels in an atom or molecule, it can cause a transition between these energy levels. This results in absorption of the photon, which transfers its energy to the atom or molecule. For example, the energy spacing between the ground and first excited electronic states of a hydrogen atom is 1.64 × 10−18 J which corresponds to a photon frequency of 2.47 × 1015 Hz. So photons with this frequency can excite the electron in a hydrogen atom from the ground to first excited electronic state.
A laser beam is simply a beam of light that has been intensified by stimulating the emission of photons. They can be used for many purposes, ranging from cutting metal to delicate surgery to aiding scientists with complex measurements.
What is a laser?
A laser is a light source that emits light amplified through the stimulated emission of photons. The acronym, LASER, actually stands for Light Amplification by Stimulated Emission of Radiation. Lasers come in many shapes and sizes. Some can fit in your pocket, and some are huge and take up entire rooms. Some emit pulses of light, while others emit continuous beams of light. Since there are so many types of lasers, it’s not surprising that they find wide-ranging applications from simply pointing at a screen during a presentation to carrying out complex measurements in physics and chemistry experiments.
Why are lasers useful for physical chemists?
Chemists use lasers to study how molecules interact with light. In some cases, a chemist may want to know how a molecule reacts when a pulse of light is used to excite it. In other cases, lasers can be used to gain information about the structure of the molecule. One of the reasons lasers are good for these purposes is that they can provide pulsed light to gain information about how molecules are changing over time. Another is that many “tricks” exist for controlling and manipulating the wavelength of the light produced by a laser, making them versatile light sources.
Can lasers be dangerous?
Definitely. Many lasers used in modern chemistry laboratories are powerful enough to cause a person to go blind after only a fraction of a second of direct exposure to the eye. Some are even so powerful that they can burn or ignite objects placed in their path. Laser pointers you can buy in the store are not this powerful, however, so you don’t have to worry about a laser you personally own being quite this dangerous. You should still definitely avoid shining them in your eye, though, because they can be damaging.
What is the biggest laser in the world?
The largest laser in the world is located at the Lawrence Livermore National Laboratory in Livermore, California. This laser is so large that it covers the size of three football fields! The scientists who use this giant laser for their research are hoping to show that a nuclear fusion reaction (see “Nuclear Chemistry”) can be controlled and used as a source of energy. If that’s possible, it could revolutionize the way power plants make energy.