The Handy Chemistry Answer Book (2014)
POLYMER CHEMISTRY
POLYMERS ARE MOLECULES TOO!
What is a polymer?
Polymers are large molecules, usually made up of smaller repeating units. The word itself, polymer, means “many parts” in Greek. You probably started thinking about plastics (like milk jugs and plastic cups) when you read the title of this chapter. Plastics are common examples, but polymers also play important roles in all plants and animals, including you.
What is a monomer?
If polymer means “many parts,” a monomer is “one part” of that whole. A monomer is a molecule that is attached to many copies of itself to make a polymer molecule. Usually these bonds are covalent, but not always.
How are polymers different than small molecules?
So many ways! Polymer chemistry and polymer physics are big areas of research both in the recent past and today because connecting a bunch of small molecules into one big one results in lots of interesting changes.
To give you a metaphor, let’s talk about pasta. Start with uncooked macaroni and uncooked spaghetti: If you try to move your hand through a bowl of uncooked macaroni you won’t have much trouble, but if you had spaghetti noodles all lined up and you tried to move your hand through them (in either direction!), you’ll run into problems. You either need to break the noodles or you need to carefully thread the noodles through your fingers. Both of these actions require energy (enthalpy in the first case and entropy in the second).
Now let’s cook those noodles. Stick a fork in each of the bowls and spin it around. With macaroni, nothing happens, but the spaghetti starts to wind around your fork, gets tangled up, and so on.
Macaroni, a collection of small molecules…I mean, noodles, is totally different than polymers (spaghetti) which are also made up of flour and water, but are much longer. The raw and cooked spaghetti aren’t just easy to imagine, they’re great ways to think about polymers in different states (solids and liquids, glassy states and polymer melts).
Are all polymer chains the same size?
No. Let’s stick with the macaroni metaphor to understand this. Imagine you’re stringing noodles together to make a macaroni necklace. You can put as many noodles on a single string as you want. If you have two strings, you can put an equal number of noodles on each string or make one longer than another. Again, the macaroni noodles are monomers, which form polymers when we string them together.
So if all polymers are not the same size, what is the weight of a polymer?
Good question. If we know the number of monomers that make up a polymer chain (technical term: degree of polymerization), then the molecular weight of the polymer is the molecular weight of the monomer multiplied by that number of monomers.
How do you measure molecular weight of a polymer?
The most common way is based on size. The technique is known as size exclusion chromatography, or gel permeation chromatography. The sample is passed through a column that has a porous solid material. The smaller polymers can work their way into those pores, while larger molecules don’t interact with the solid material. The biggest molecules, because they don’t interact with the solid phase, come out of the column first followed by smaller and smaller molecules. The time it takes for a polymer to get through the porous column is related to its molecular weight (okay, technically it’s based on the hydrodynamic volume, but let’s let this approximation slide). In practice, these instruments are calibrated using standard polymer samples of a known molecular weight.
What is molecular weight distribution?
We just talked about the fact that polymers can have different molecular weights. Oftentimes in reactions that make polymers a range of molecular weights are produced. The molecules may be composed of the same repeating unit (monomer), but for a number of reasons the chains are different in length. It turns out that this distribution of lengths is important to a number of polymer properties. The details of how this number is calculated are not worth going into; it’s sufficient to know that a higher molecular weight distribution means that there is a larger spread of polymer chain lengths. A distribution of 1.0 would mean that every single polymer chain has the exact same molecular weight.
Does polymer stereochemistry matter?
In lots of examples, it does. Let’s stick with polypropylene for now. Isotactic polypropylene is a crystalline material with a melting point around 160 °C. The crystallinity is due to the perfect arrangement of the methyl groups along the polymer backbone. This crystallinity makes the material very tough, so it’s used in all sorts of applications—from pipes to plastic chairs and carpeting. But if there are errors along the polymer chain (methyl groups pointing in the wrong direction) the melting point decreases, and the plastic loses its strength.
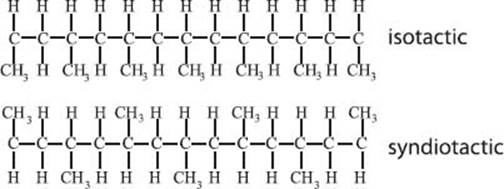
Do polymers have stereochemistry like small molecules?
Yes! The most common example is polypropylene. This polymer has a methyl group attached to the backbone of the polymer. If the methyl groups are all on the same side of the chain, the stereochemistry is known as isotactic (top structure below). If the arrangement of the methyl groups alternates which side of the chain it’s on, the polymer is called syndiotactic (bottom structure below). If there’s no order at all to the substituents, we call the polymer atactic.
Are all polymers linear chains?
No, and this is another way that chemists classify these really big molecules. The major types of polymer shapes (technical term: topology) are linear, branched, and crosslinked networks. Linear polymers are chains of monomers joined together, like a noodle or a rope. If there is a point along a polymer chain where a second chain starts, like a fork in the road, this arrangement is referred to as branched.
What is a crosslinked polymer?
When a bond is formed between two polymer chains (and technically not at the chain ends), the product is called a crosslinked polymer. Creating linkages between chains usually increases their viscosity (so more like molasses than olive oil) and creates elastic properties like those found in rubber bands. At higher levels of crosslinking, polymers can even become stiff or glassy.