The Handy Chemistry Answer Book (2014)
POLYMER CHEMISTRY
POLYMERS IN AND AROUND YOU
What polymers are found in nature?
There are a ton! Proteins, enzymes, cellulose, starch, and silk are all polymers.
Is DNA a polymer?
It is. DNA contains two long polymers of sugars (called nucleotides). Attached to each sugar molecule are phosphate groups and a nitrogen base (technically a nucleobase). The sequence of these nucleobases encodes the information in DNA. (For more on DNA, se the “Biochemistry” chapter.)
What is cellulose?
Cellulose (see diagram below) is linear polysaccharide—“poly” for many and “saccharide” means sugar, so cellulose is a chain of sugar molecules. It’s an amazing molecule: it’s the most abundant organic compound on the planet because it is the main component in plant cell walls. Cellulose is highly crystalline because of the way the sugar molecules are connected and because of the fact that it’s made up of a single enantiomer of glucose. Like polypropylene, highly crystalline polymers like cellulose are very strong—strong enough to make trees stand up straight.
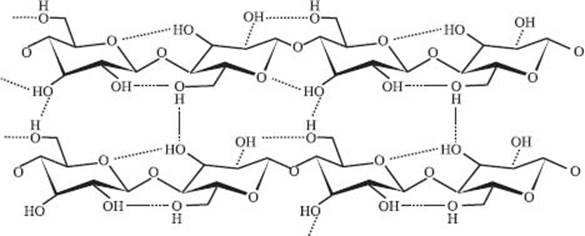
Is starch different than cellulose?
Starch is also a polysaccharide, like cellulose, but it is much less crystalline. The major component of starch is amylopectin, which is a highly branched polymer, while cellulose is strictly linear. These branches prevent amylopectin from crystallizing as well as cellulose. Starch is an excellent source of energy (or stored sugar) for plants and animals for these reasons: Because it is less crystalline than cellulose it is more soluble than cellulose, and the branched structure also means there are more end groups at which enzymes can start “chewing” the polymer apart.
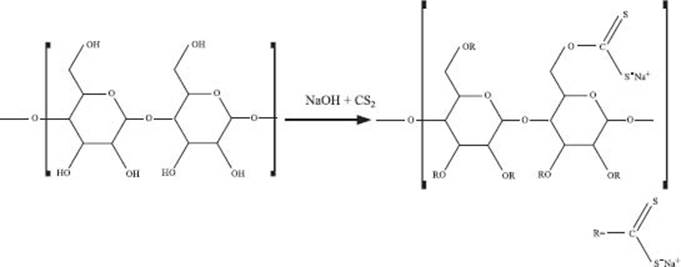
What is rayon?
You probably know what rayon fabric looks or feels like—the best Hawaiian shirts (and much of 1980s fashion) were made of it. What’s fascinating about rayon is that it is not really a synthetic or a natural fiber. Rayon is a chemically modified cellulose polymer, first prepared in the 1850s. While there have been a number of ways of preparing this “artificial silk,” the Viscose method led to the first commercial production of rayon. This method treated cellulose with a combination of sodium hydroxide and carbon disulfide as indicated below.
How was rayon discovered?
The first artificial silk was probably prepared by a Swiss chemist, Georges Audemars, in 1855. Audemars mixed the pulp of mulberry bark (chosen likely because silkworms eat mulberry leaves) and a rubber gum and used a needle to pull out long fibers of material. This was a rather labor-intensive and difficult process and could not be done in any economic way. Some accounts also claim that Audemars drew fibers of nitrocellulose (the product of mixing nitric acid with cellulose); in addition to being a delicate process, the resulting fibers of nitrocellulose were highly flammable.
Hilaire de Chardonnet, a French engineer, was another key player in the history of artificial silk. Working with Louis Pasteur in the 1870s, the legend claims that he spilled a bottle of nitrocellulose while working in a photography darkroom. The spilled solution was left to evaporate, and Chardonnet returned later to clean up his mess. Wiping up the residue, he noticed long, thin fibers had formed. Chardonnet received a patent on this material, but again the flammability kept it from achieving large market adoption.
The Viscose method mentioned earlier was finally worked out in 1894 by English chemists Charles Frederick Cross, Edward John Bevan, and Clayton Beadle. This method was a commercial success, and the fabric was manufactured first by Courtaulds Fibers in the United Kingdom and then Avtex Fibers in the United States.
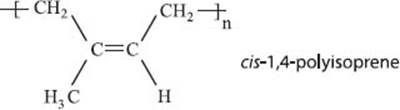
Where does rubber come from?
Rubber trees! No kidding. Natural rubber is collected from rubber trees like maple syrup comes from maple trees, except the syrup is latex sap. It’s a polymer of isoprene where each carbon-carbon double bond along the chain has cis-stereochemistry. Although there are man-made alternatives produced artificially, even today about half of the rubber produced each year on our planet does come from rubber trees.
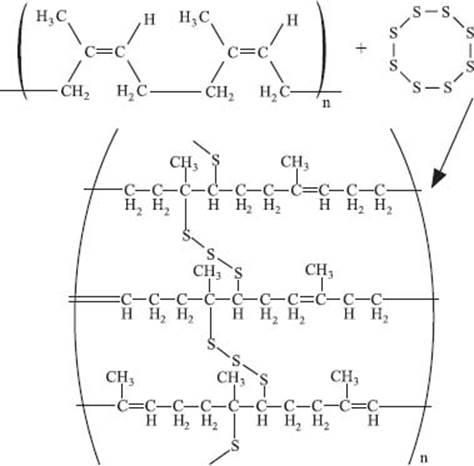
What is vulcanization?
Natural rubber that comes directly from rubber trees looks nothing like your car or bike tires. It’s sticky, doesn’t hold its shape when it gets warm, and if you live where it snows, it can get brittle. That sounds like an awful material to make a tire out of! Vulcanization creates crosslinks in the rubber with the addition of sulfur to the natural rubber chains and improves all of these properties.
What is an addition polymerization reaction?
The easiest way to describe an addition polymerization reaction is that monomers are bonded together without the loss of any atoms of the monomer. There are more complicated ways to classify this type of reaction based on kinetics, but it essentially boils down to this fact.
How is a condensation polymerization reaction different?
Addition polymerization reactions do not involve the loss of any atoms from the monomer, but condensation polymerization reactions do. The molecule that is lost is almost always water.
What do the recycle numbers mean on plastic bottles?
These are technically called Resin Identification Codes (RSI) and were introduced in the 1980s to make it easy to separate plastics for recycling. The numbers correspond to what kind of polymer the item is made of and have no other meaning. They are not in any sort of order based on how easy or hard it is to recycle the resins, despite rumors you might have heard about this.
|
RSI |
Plastic |
|
1 |
Polyethylene terephthalate |
|
2 |
High-density polyethylene |
|
3 |
Polyvinylchloride |
|
4 |
Low-density polyethylene |
|
5 |
Polypropylene |
|
6 |
Polystyrene |
|
7 |
All others |
What is a thermoplastic?
If a polymer becomes soft when it is heated (and then hardens again when cooled), it’s known as a thermoplastic. The temperature at which the polymer softens depends on what it’s made of and on the size of the polymer chains. Thermoplastics are easy to recycle because they can be remolded when they’re hot.
What is a thermoset?
Unlike a thermoplastic, thermoset materials are cured such that they don’t soften when exposed to heat (at least up to a certain point). This curing step usually introduces a lot of crosslinks between polymer chains, which creates a rigid network. These materials are much more difficult to recycle, but are used where high-temperature stability is needed.

According to the EPA, in 2010 Americans only recycled eight percent of their plastic waste. We can do better. About seventy-five percent of the packaging we use is recyclable.
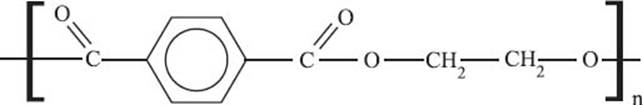
What is PET (polyethylene terephthalate)?
Polyethylene terephthalate is a thermoplastic that is used in both textiles, where it’s called polyester, and in bottling, where the same polymer is called PET. PET is an alternating polymer of ethylene and terephthalate monomers. This material is really good at preventing gases from diffusing through it, so it’s great for keeping carbonated drinks fizzy.
What is HDPE (high-density polyethylene)?
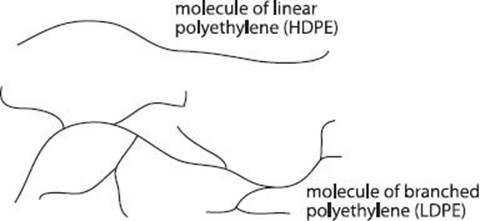
High-density polyethylene is technically any polyethylene with a density of 0.93 to 0.97 g/cm3. The density in polyethylene is controlled mostly by the number of branch points along the polymer chain. HDPE has very few branches, so the chains can stack together very closely. This tight packing makes it a very strong polymer, so it’s used to make things like bottlecaps, milk jugs, and Hula Hoops.
What is LDPE (low-density polyethylene)?
If the density of a polyethylene is between 0.91 to 0.94 g/cm3 (yes, there’s a little overlap in these ranges) it’s called low-density polyethylene. To get to this density, the polyethylene chains have more branching than in HDPE but still only a few percent of the atoms along an entire chain. These branches prevent the chains from stacking together quite as well, which makes the material softer and more flexible. With those properties, LDPE finds use as trash, grocery, and sandwich bags, and that “clingy” food wrap (although the original Saran® Wrap was not LDPE—see below).
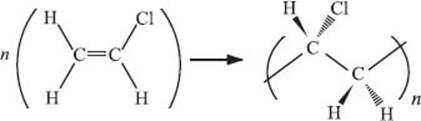
What is PVC (polyvinyl chloride)?
If one of the hydrogen atoms on every ethylene monomer in polyethylene is replaced by a chlorine atom (note that this is not how this material is actually made!), you get PVC, or polyvinylchloride. It’s the third-largest-volume polymer produced each year behind polyethylene and polypropylene. It is a very tough polymer, so it is used to make pipes and flooring among many other things. PVC can also be softened (technical term: plasticized) by introducing small organic molecules, like phthalates (a benzene ring with two esters). Among other applications, plasticized PVC is used to insulate electric wires and to make your garden hose.
What is my credit card made of?
Also PVC—but for the material in credit cards, no plasticizer is added. To manufacture a credit card, usually a few thin sheets of PVC are glued together.
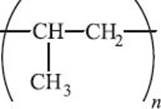
What is PP (polypropylene)?
If instead of substituting a chlorine atom we add a methyl group to each ethylene monomer, we get polypropylene. Recall from earlier that this introduces stereochemistry along the polymer. We’ve already mentioned that the arrangement of the methyl groups along the polymer chain can have large effects on the melting point and other physical properties. You can likely find polypropylene all over your house from dishwasher-safe food containers to synthetic carpets (especially outdoor carpeting) and an increasing weight fraction of your car, including the bumper and the casing for the battery. It can also be made into ropes, which are quite strong and resistant to weather, so they are frequently used in fishing and farming. Polypropylene is also used for many medical applications because it is capable of withstanding the high temperatures required to sterilize.
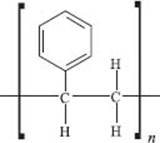
What is PS (polystyrene)?
If one of the hydrogen atoms on every ethylene monomer in polyethylene is replaced by a benzene ring (note again that this is not how this material is actually made!), you get PS, or polystyrene. Polystyrene is usually the fourth-largest-volume polymer produced globally, with billions of pounds made annually. Polystyrene can be manufactured into parts (like CD cases, furniture, and eating utensils), or air can be mixed with the polymer to make a foam used in insulation both for your house and your coffee cup. Styrofoam® is a trademarked name held by the Dow Chemical Company for foamed polystyrene.
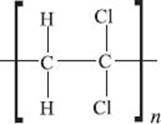
What is Saran® Wrap?
Saran® Wrap is a trade name (another held by the Dow Chemical Company) for polyvinylidene chloride. If two of the hydrogen atoms on every other carbon in polyethylene are replaced by chlorine atoms (note yet again that this is not how this material is actually made!), you get PVDC, or polyvinylidene chloride. It was discovered by accident in 1933 by Ralph Wiley, who was having trouble washing this strange material out of the bottom of a piece of glassware. The actual polymer they were trying to make was poly(perchloroethylene)—where every hydrogen is replaced by a chlorine atom. It was just before WWII that a breakthrough was made that allowed the scientists to make film from this new material. It was quickly adopted by the Army to wrap equipment being transported by sea in order to prevent corrosion from saltwater and other applications to keep soldiers dry in jungle environments. After WWII, Dow found a new use for the material and introduced a PVDC film product for wrapping food called Saran® Wrap. The clingy food wrap you buy today is not PVDC, however. This material was phased out due to environmental and health concerns of those chlorine atoms, and low-density polyethylene took its place.
Okay, but why was PVDC ever called Saran® Wrap?
Many industrial trade names have no interesting story behind the creation. Saran® Wrap is an exception. You might think that Ralph Wiley was responsible for naming this material, having discovered it. Nope. Ralph Wiley’s boss, John Reilly, named the material after his wife and daughter—Sarah and Ann.
What is nylon?
Nylon is a synthetic polymer formed by the condensation of a dicarboxylic acid with a diamine. This reaction forms an amide bond and releases a molecule of water. The term “nylon” is a generic name for these types of polymers, but one of the common nylons is called “Nylon 66.” The numbers signify the number of carbon atoms in the amine (6) and the acid (6) reactants.
What makes my cooking pans “nonstick”?
The coating that is placed on cookware is usually polytetrafluoroethylene (PTFE), marketed as Teflon® by DuPont®. The strength of the C-F bond, and its reluctance to interact with just about anything else, makes Teflon® very heat-resistant and slippery stuff. Aside from coating cookware, it’s used to make gears and bearings, and it’s a key component of Gore-Tex (the material your waterproof jacket is made of).
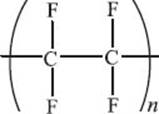
When was nylon discovered?
Nylon 66 was first made by Wallace Carothers, a scientist working at DuPont®, on February 28, 1935. Dr. Carothers also contributed to the discovery of neoprene, which is used to make suits used for scuba diving.
What was nylon first used for?
The first commercial application was probably toothbrush bristles. For centuries toothbrushes were made of coarse animal hairs (usually boar) until Dupont® introduced Doctor West’s Miracle Toothbrush in 1938.
What is silicone? Is that the same as silicon?
Silicon is an element, while silicone is a polymer with a backbone of silicon and oxygen atoms. These polymers are very resistant to heat and have a rubbery feel. The latest squishy cookware and bakeware is made out of silicone.
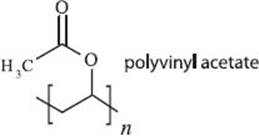
What is glue?
There are many different types of adhesives, but you’re probably thinking of that white glue you had as a kid. This type of glue is known as a “drying adhesive” because it hardens by evaporation of a solvent. In the case of white glue, the solvent is water and the sticky stuff that gets left behind is polyvinyl acetate.
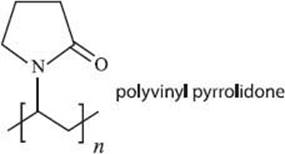
Does hairspray contain polymers?
Yep—and almost the same ones that make white glue and acrylic paints. Many hairsprays contain vinyl acetate (or something similar), polyvinyl pyrrolidone, and/or lots of other variants. Like glue, you also need a liquid to disperse or dissolve the polymers, but in hairspray a mixture of alcohol and water is normally used.
What’s in paint?
There are three main ingredients in paint: binder, solvent, and pigment. Binder is the stuff in paint that forms an adhesive so that it sticks to the wall. Unlike glue, it’s not a polymer in paint (at least not a fully formed one), but instead monomers or short polymer chains that react (crosslink) to form larger polymer networks as the solvent evaporates. The solvent in paint is there to make the paint solution the right thickness so that you can easily put it on the wall without it dripping all over the floor. Then the solvent evaporates, driving the formation of the crosslinked polymer networks. Pigments are, of course, used in paint so that not everything is painted white (though white paint still needs pigments to be white!).
How is recycled plastic used to make fleece?
Fleece can be made from polyethylene terephthalate (PET) bottles. The first step is to wash and then mechanically crush the bottles, shaping the plastic into small chips. The chips can then be heated and forced through tiny holes in a metal plate (called a spinneret), which forms fibers that harden as they cool to room temperature. These fibers are wound onto a spool as they are formed, and they can subsequently be stretched to improve their strength. Machines can then be used to texturize and cut the fibers to their desired length and be used to make fleece cloth for clothing, blankets, etc.
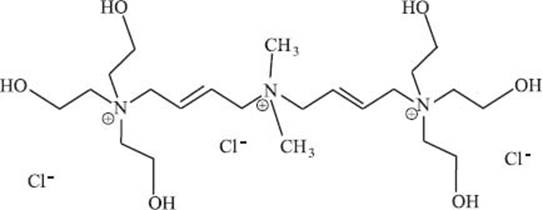
There are polymers in my shampoo and conditioner, too?
There are! Many ingredients in shampoos and conditioners are similar to most soaps (surfactants, etc.), but cationic polymers play important roles in these products. One family of these polymers is named “polyquaterniums,” which is closer to a trade name than a technical chemistry name (the structure of one member of this family, “polyquaternium 1,” is shown below). All of these polymers contain positive charges, which allows them to form ionic bonds with hair strands. This prevents the polymers from being rinsed away with water. Once coated, your hair strands are now less likely to stick to adjacent strands and appear shinier.
What is fiberglass?
Fiberglass is a polymer made from a plastic matrix that is reinforced by fibers of glass. It is a popular material because it is inexpensive to make, and its strength and weight properties compare favorably against those of metals for many applications. Fiberglass has a wide range of applications and is commonly used in glider aircraft, boats, cars, showers and bathtubs, roofs, pipes, and surfboards.
What absorbs liquid in diapers?
The general term for the materials that absorb water in diapers is “super-absorbent polymers”; these same compounds are used to chill drinks and to make fire retardants and fake snow. Modern absorbing materials are typically sodium salts of polyacrylic acids, and these can become almost entirely water by weight and as much as 30–60% by volume.
Wait—absorbent polymers are also used to chill drinks? How does that work?
If you stuff a cup holder with absorbent polymer and then soak it in water, the polymer will obviously swell up. The water will slowly evaporate out of the polymer, which reduces the temperature of the polymer gel and ultimately your drink.
What is Styrofoam®?
Styrofoam® is a brand name (owned by the Dow Chemical Company) for expanded polystyrene foam. It’s 98% air, which is why those styrofoam® coffee cups are so light (and actually buoyant). In addition to disposable food containers, polystyrene foam is used in building and pipe insulation, packing peanuts, and that green stuff they use for holding together fake flower arrangements.
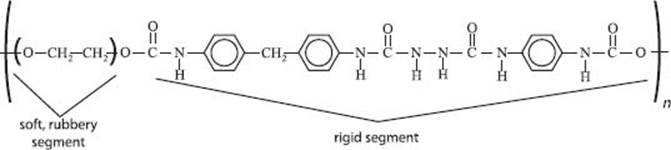
And spandex? What’s that?
Spandex (as it’s called in North America; Europe knows the same material as “elastane” and the Brits refer to it as “lycra,” but that’s confusing because Lycra® is actually a trade name) is a rigid copolymer of polyurethane and polyurea and is a rubbery material, like polypropylene oxide. These two polymers do not mix well, so tiny domains of each polymer form. It’s this separation (of the hard bits from the soft bits) that gives Spandex its stretchy, yet strong, behavior.