The Handy Chemistry Answer Book (2014)
CHEMISTRY IN THE KITCHEN

Do chemists really study food chemistry?
Yes, they really do! There’s even a scientific journal called Food Chemistry (published by Elsevier) dedicated to reporting new findings regarding the chemistry and biochemistry of food and raw (food) materials.
What is the Maillard reaction?
The Maillard reaction is technically “nonenzymatic browning,” which basically includes any kind of browning that happens when you’re cooking, but excludes what happens to cut apples you leave out on the counter. At the chemical level, it’s a reaction between an amino acid and a sugar in the presence of heat. A huge number of chemicals are formed in these processes, so you can’t really pin down the Maillard reaction to a single set of chemical steps. These processes are responsible for the browning of meat, the malting of barley for beer, the roasting of coffee, and the browning of the crust of bread.
Is caramelization the same thing as the Maillard reaction?
Caramelization is the breakdown of sugar molecules with heat (pyrolysis), while the Maillard reaction requires amino acids (proteins). Caramelization, like the Maillard reaction, is a term for hundreds of different chemical reactions taking place at the same time.
How does baking soda make my cookies better?
Baking soda is sodium bicarbonate (NaHCO3) and is used in cooking as a leavening agent (as in, it helps things to rise). It does this by releasing carbon dioxide (CO2), which it does in the presence of acids, like buttermilk, vinegar, lemon juice, cream of tartar, and so on. The process is much faster at higher temperatures. Once you put your cookies in the oven, the sodium bicarbonate begins to break down and the carbon dioxide that is released makes tiny little bubbles in the batter. These tiny bubbles get trapped as the cookies bake, making them light and fluffy.
What’s the difference between baking soda and baking powder?
Baking powder is a mixture of three things: baking soda, an acid, and a filler—usually cornstarch. The acid takes the place of the buttermilk or lemon juice in a recipe (see the previous question) to release CO2 from NaHCO3. The starch is in the mix to keep the two components from reacting before it gets into your cookies and to keep everything dry.
If baking soda and baking powder are different, how can I substitute one for another?
Since baking powder is diluted baking soda with acid, if your recipe calls for powder but you only have soda, you’ll need to use less, but add an acid. Generally the ratio is three parts baking powder equals one part baking soda with two parts cream of tartar (or another acid substitute).
What chemicals are used to preserve food?
There are two main types of food preservatives, those that prevent oxidation and those that prevent bacteria or fungi from growing. The first are appropriately named antioxidants, and these molecules work by reacting with oxygen themselves. Unsaturated fats are common targets of oxygen, which causes foods to become rancid. Antioxidants provide an even easier target for oxygen to attack, preventing O2 from wreaking havoc in other ways. Natural antioxidants include molecules like ascorbic acid (vitamin C); and there are also many unnatural antioxidants on the market.
The second class of preservatives are those that stop the growth of bacteria and fungi (like mold) from growing. Many of these preservatives are acidic molecules that can be absorbed into the cells of bacteria. If enough acid gets into the cell, basic biochemical functions (specifically fermentation of glucose) slow down enough that the cell dies, and your food stays fresh.
What is cream of tartar?
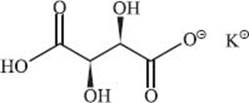
Cream of tartar is the potassium acid salt of tartaric acid. This means one acidic hydrogen of tartaric acid is replaced with a potassium ion. The structure is shown below. If you’ve ever seen crystals of something in a bottle of wine (or even fresh grape juice), it was probably this chemical.
What’s in Jell-O® that makes it so jiggly?
Gelatin, which is a convenient and pleasant term for the mixture of proteins and shorter peptides that are obtained from boiling byproducts of the meat and leather industries (mostly bones and pig skin), makes Jell-O® jiggly. The hydrogen bonds between peptide strands allows gelatin to form a network in the presence of water, which is what the physical structure of the gel is that you are familiar with. These hydrogen bonds can be disrupted with heat, which is why you boil Jell-O® before pouring it into a mold; once it cools back down, the network reforms in whatever shape the liquid is in.

Gelatin, the main ingredient in Jell-O® desserts, is made up of proteins and peptides extracted from boiling leather and meat byproducts. Yummm.
How does pectin gel make jelly?
Pectin is a polysaccharide from the cell walls of plants that helps plant cells to grow and also to stick to their neighbors. Commercially, pectin is extracted mostly from citrus peels, but other plants are also used. Pectin is the gelling agent that helps jams and jellies to set, giving them a consistency that is useful for spreading on toast. It does this using a very similar mechanism as the one it uses to keep plant cells together. The chains of sugar molecules (polysaccharides) can form bond between strands, creating an elastic network known as a gel…or jelly.
Why do some fruits and vegetables turn brown after you cut them?
When you cut or drop an apple, potato, or other fruit, some cells leak their contents, exposing all sorts of cellular machinery to air. The key player in the browning of fruits is tyrosinase, an enzyme that is involved in the oxidation of tyrosine specifically and phenols in general. Tyrosinase plays two key roles in the production of melanin, which is a general term for all sorts of pigments found in plants and animals. So once tyrosinase leaks out of the cell, it is exposed to all the oxygen and phenols it needs to start making brown pigments.
Why does lemon juice prevent fruits from browning?
If you put lemon juice on your fruit after cutting it to prevent “browning,” this is an example of that last category of preservative—one that slows some enzymatic process. The vitamin C lowers the pH (because it is an acid), slowing the enzymes (polyphenol oxidases and tyrosinases) that cause browning of your fruit.
Why do onions make you cry?
Sulfinylpropene is the main tear-inducing compound (technical term: lachrymator) released when you slice onions. The most interesting part of this story is that this chemical is not present in onions before you slice them. In fact, the generation of this tear-inducing molecule is not a mistake, but part of the defense system that plants have developed to deter animals from continuing to eat them. When you cut, or an animal takes a bite of, an onion, an enzyme, alliinase, that is normally safely stored within the plant’s cells is released and starts to wreak havoc. Alliinase converts sulfoxides to sulfinyl groups, which makes you cry like a baby.
So is there any real way to stop onions from making you cry?
Everyone’s mother seems to have their own unique proposal about how to avoid crying over diced onions, but there’s only one that makes any chemical sense (excluding wearing a gas mask): put the onion in the fridge before you cut it. Almost every chemical reaction is slower at lower temperature, and since the lachrymator in onions is produced when you cut it (and not naturally present in the onion), you can give yourself more tear-free time to dice if the onion is cold.
How is refined sugar different from raw sugar?
Refined sugar is raw sugar that has been purified by a series of steps that ends with crystallization of a sugar syrup into white sugar crystals. The debate of whether refined sugar is worse for you than raw sugar is ongoing, but chemically refined sugar is just more pure sugar than raw sugar.
What about beet and cane sugar, how are those different?
Beet sugar comes from sugar beets. Cane sugar comes from sugarcane. After these two sugars are purified (refined), there is no chemical difference.
So what is molasses, then?
Molasses is the byproduct of refining sugarcane. In one of the crystallization steps, the brown liquid that is left behind is concentrated into a syrup known as molasses. The molasses you may have used in cooking or baking is from sugarcane. Sugar beet molasses has a lot of other chemicals and it tastes terrible to us; not everyone seems to mind though—it’s a common additive to animal feed.
What is Splenda®?
Splenda® is a commercial name for an artificial sweetener, like NutraSweet® or Equal®. Sucralose is the key sweet ingredient in Splenda®, but unlike natural sugar molecules, sucralose is not metabolized, so it effectively has zero calories. It’s made from regular table sugar by selectively exchanging three OH groups for chlorine atoms.
What is Stevia?
Stevia is a commercial name for an artificial sweetener and is also the common name for the plant that it is extracted from. Steviol is the basic structure of this class of sweetener, but when sugar molecules are attached to steviol (making it steviol glycoside) its sweetness skyrockets to hundreds of times that of regular sugar. This sweetener has been in use for centuries in South and Central America and in Japan since the 1970s. In the United States, it’s only been available for a few years as a purified compound (marketed under the name Truvia®); raw plant extracts of the stevia plant are not approved for use in the United States.
Okay—last sweetener question—what is corn syrup?
Corn syrup is made from cornstarch either by heating up starch in an acidic water solution or by adding an enzyme to break down the long starch molecules into simpler sugars. Chemically it is mostly maltose, which is a disaccharide (two glucose molecules stuck together) along with a small amount of larger chains of sugar molecules (oligosaccharides). The high-fructose variety is made by treating regular corn syrup with a second enzyme that converts glucose into (you guessed it) fructose.
What does brining do to meat?
Soaking meat in a salt solution (the definition of brining) helps separate the long filaments that make up muscles (myofibril) by dissolving the proteins on their surfaces. With enough salt the actual filaments start to break down, and both effects make the meat seem more tender. Additionally salt allows proteins to hold on to more water, which helps prevent your steak from drying out.
What is removed from butter to make clarified butter?
Proteins and water. Clarified butter is made by melting regular butter at a low temperature. Three layers will form: the top frothy layer contains the proteins from milk (casein, used to make cheese); the middle layer is water with dissolved milk sugars, like lactose; the bottom layer is pure butterfat or milkfat, which is also known as clarified butter. You can instead heat butter at a low temperature for a long time to remove the water by evaporation, and then decant or filter the butterfat. Clarified butter contains almost no proteins, so it has a very long shelf life, and no lactose, so people who are lactose-intolerant can eat it.
How does nonstick cooking spray work?
It’s not nearly as magical as you might think. Cooking spray is just regular vegetable oil in spray form. To get it to spray out a fine mist, an emulsifier is added, and the can needs something to act as a propellant (usually alcohol, CO2, or propane).
If cooking spray is just oil, then how can it have zero calories and no fat?
Cooking spray lets you apply a thinner layer of oil than you could probably achieve by pouring normal liquid vegetable oil out of a bottle. The FDA states that any food substance with less than five calories and less than 0.5 g of fat per serving can be labeled calorie-free and fat-free, respectively. So manufacturers of cooking spray adjust the recommended serving size to contain less than those limits. As a result, your can of spray contains hundreds of servings—go check your pantry if you don’t believe it!
How do instant noodles cook so fast?
Because they’re already cooked! Instant noodles were invented in Japan in the year 1958 by Momofuku Ando, who was working at Nissin Foods. The noodles are flash fried, creating a dry noodle with a very long shelf life that can be prepared in minutes.
What is homogenized milk?
Homogenized milk is milk that won’t separate. Normally cream will separate out from milk, forming a layer at the top of the bottle. This is obviously not ideal, so to prevent this separation from happening, milk is treated with pressure to break up the little clusters of fat into much, much tinier pieces. These tiny globules of fat don’t recombine at an appreciable rate, so the milk remains a single layer throughout its shelf life.

Instant noodles are pre-cooked and dried by flash frying. In this way, they can be stored for a long time and quickly cooked in hot water.
What makes fish smell fishy?
Fresh fish doesn’t smell fishy at all. It’s only when proteins and amino acids in fish start to break down, releasing stinky nitrogen and sulfur compounds, that the funk sets in. There are a few reasons that this sort of smelly decay is more common with fish than with chicken, beef, or pork. Fish frequently eat other fish, so their digestive systems contains enzymes that can break down the proteins found in fish. So if some of these enzymes leak out, or if you’re slow to gut your fish, those enzymes will go to work…on its own flesh. Fish also generally have higher levels of unsaturated fats, which are less stable than saturated fats to oxidation. Acids, like lemon juice, can slow the enzymes down, and convert the amines into less odorous ammonium salts, which is probably why we’re all used to squeezing a lemon wedge on fish.
Why does elevation matter for cooking times when I’m boiling water?
If you’re boiling water in Denver, the temperature of that water will be about 5 °C lower than if you were boiling water in Miami. Because Denver is about a mile above sea level, there is less atmosphere pushing down on that pot of water than there would be at sea level. The decreased temperature that water boils at means that you’ll need to increase cooking times the higher up you go.
How does a pressure cooker speed up cooking?
If the lower atmospheric pressure in Denver increased cooking times by lowering the boiling point of water, what if we could increase the boiling point of water? That’s exactly what a pressure cooker does. Pressure cookers are sealed such that once you start to heat water, the pressure inside the vessel increases. This increase in pressure drives up the boiling point of water because every water molecule that tries to make the transition from water to liquid has a greater force pushing against it. By increasing the pressure inside the pot, pressure cookers can get the boiling point of water up to about 120 °C (250 °F). With water boiling at a higher temperature, your food cooks faster.
Does hot water really freeze faster than cold water?
Sometimes. This observation is known as the “Mpemba effect,” named after the Tanzanian student who in 1963 resurrected the idea from Aristotle, Francis Bacon, and René Descartes. Whether or not this effect can be seen seems to depend on so many variables (the size and shape of the container, the initial temperatures of the two liquids, the method of cooling), on how you define freezing (when the first ice crystal forms, when there’s a solid layer on the top, or when all of the water has frozen solid), that it’s really still unclear if this effect is real or not.
Does all the alcohol really boil off when I cook with wine?
Not really, no. It’s common lore that when you add red wine to pasta sauce that the alcohol evaporates rather quickly. This idea is supported by the fact that alcohol has a lower boiling point than water, so it should evaporate quickly. People who actually studied this, however, have shown that even after an hour, 25% of the alcohol you added is still in the sauce. If you want a truly nonalcoholic marinara, you need to simmer for at least two and a half hours.

When improperly packaged food is put into a freezer, water can be drawn out of the food and crystalize; oxidation can also occur. It is still safe to eat the food, though it is not as appetizing.
What is freezer burn?
Freezer burn occurs when frozen food undergoes dehydration due to improper packaging. The humidity level in a freezer is usually quite low, so if food is not stored in airtight packaging, water in the food can be drawn out into the freezer atmosphere by sublimation. Also, because the food is exposed to air, oxidation can occur, though at the lower temperatures in a freezer, these reactions are quite slow. Thankfully, although freezer burn looks nasty it’s not a food safety concern, it just causes discoloration.
Why can’t I put raw eggs in the freezer?
You can if you take them out of their shells. Raw eggs expand when frozen, which can break the shell, so don’t put whole eggs directly into the freezer.
Are green, oolong, and black teas made from different plants?
No, all tea is made from the leaves of a single plant, Camellia sinensis. That statement excludes herbal teas, though, which are more accurately called infusions. Different categories of tea are prepared using different processes of wilting, bruising, and drying the leaves. Green tea is processed within a day or two of harvest, which preserves the natural chemicals of the fresh leaves. Black tea leaves are prepared by an oxidation process at high temperature and humidity, and then dried. Oolong tea is in between green and black: the leaves are left for a few days to wither, after which a short oxidation process is performed.
What makes a bowl microwave safe?
Unfortunately, the only definition is an empirical one: containers that don’t get hot in the microwave are microwave safe. Remember (or go look it up now in “The World Around Us”) that microwaves heat food by causing molecules with dipole moments to tumble back and forth. If the container has any such molecules, it’ll get hot in the microwave. If any water has leached into your ceramic mug, it’ll get hot in the microwave. Regardless of whether the container gets hot on its own, the food you are heating up will get hot and transfer that heat to the bowl, so be careful when taking hot things out of the microwave.
What makes string cheese stringy?
In the United States, string cheese is usually mozzarella (sometimes with some cheddar thrown in). The production process takes melted cheese and stretches and folds it in a single direction. This stretching aligns the proteins in the cheese, making it possible to peel off long strings of it.
What’s the difference between brown and white eggs?
Besides their color, the only difference is the color of the chicken that laid it. White-feathered chickens lay white eggs; brown-feathered chickens lay brown eggs. That’s it. The inside of the eggs are identical in every way, assuming the chickens you’re comparing were on identical diets.
Why do hard-boiled eggs spin, while raw eggs don’t?
Hard-boiled eggs are solid all the way through so when you spin the egg, all the energy you apply goes into spinning the whole object. Raw eggs have yolks that are free to move about the interior of the egg, however. So when you spin a raw egg the yolk moves to the outer edge of the inside of the egg, which consumes some of the energy you applied. The other difference you can see is what happens after you stop these two eggs from spinning. Stopping the hard-boiling egg stops the entire system, because the yolk is trapped in the solid, cooked egg white. Inside the raw egg, however, even after you stop it, the yolk is still spinning around. So if you take your finger off of a raw egg that was just spinning, it can start moving again, seemingly on its own.
Why do hard-boiled egg yolks turn green?
When egg whites cook, a small amount of hydrogen sulfide (H2S) is released from sulfur-containing amino acids like cystine or methionine. If the H2S migrates to the yolk, it combines with iron atoms to produce ferrous sulfide (FeS), which is a dark-colored material that looks green mixed with the bright yellow yolk. Ferric sulfide (Fe2S3) is also sometimes claimed to be formed in this process, likely because it is itself a yellow-green substance. There is, however, less data to back this up, aside from the convenient color match.
What’s in self-rising flour that makes it rise?
Baking powder and salt are added to flour to magically transform it into “self-rising flour.” Yeah, not so magical after all.
Why is there lime in my tortillas?
First, let’s clarify the question: we’re talking about traditional corn tortillas, and by lime we’re referring to a calcium hydroxide solution, not the green fruit. Corn tortillas were historically made from what is called nixtamalized corn, which involves soaking corn in a basic solution like calcium hydroxide. The process goes back about three thousand years to the Aztec and Mayan civilizations. Because of its ancient origin, how it was discovered isn’t clear, but why it survived is now obvious. Corn lacks one of the essential vitamins required in human diets; it doesn’t have niacin, also called vitamin B3. People who don’t get enough niacin in their diet develop a disease known as pellegra (just like if you lack vitamin C, you get scurvy), which has some awful symptoms. Obviously this is a problem if your civilization’s staple food is corn. Somehow, the Aztec or Mayan people figured out that cooking corn with strong bases prevented people from getting pellegra. Now we know this is because niacin is not readily available in corn, but can be released by treatment with a strong base.
Why would anyone add carbon monoxide to tuna?
Cutting a tuna fish exposes many muscle cells to oxygen, which slowly changes the bright red color of fresh tuna steaks to a darker brown. This is the result of iron-containing enzymes (myoglobin and hemoglobin) being oxidized from Fe(II) to Fe(III). Sushi lovers have come to understand that fresh raw tuna should be bright red and are skeptical of eating any brown-colored fish. The seafood industry figured out at some point that adding CO during the packaging step not only slows the rate of oxidation down, which increases shelf life, but also brightens the red color. It does this because CO binds more strongly to the Fe(II) center than Fe(III). The risk to consumers is not CO exposure here, but that you might be fooled into eating fish that isn’t as fresh as you might otherwise think if you judge its freshness based on color.
What is liquid smoke?
It’s actually exactly what it sounds like, as crazy as that is. Smoke from a wood-burning fire is blown into condensers, which collect many of the volatile chemicals in smoke. This mixture is then diluted with water. It’s used to cure bacon and flavor many other foods.
Is ceviche really “cooked” by lime juice?
The results of cooking with heat and with acid are similar, but of course they are not exactly the same. Both heat and acid serve to denature proteins in food, which is a technical way of saying that the molecules change shape. With access to any number of shapes that it couldn’t exist in beforehand, the molecule finds new ways to react both with itself and with other protein molecules. These recently liberated proteins quickly form a solid network, which is why fish gets firmer and whiter when you add lime juice, and it’s the same reason that egg whites turn opaque and get harder upon cooking.

Raw shrimp like this is grayish in color, but when cooked turns a bright pink because a red-colored molecule called astaxanthin remains in the shrimp even after less-stable pigments break up upon being heated.
Why do shrimp change color when they’re cooked?
Some, but not all shrimp, are grayish when they are raw, but turn pink once they’re cooked. It makes sense to guess that this is because some chemical compound with a red color is being produced once you add heat. What’s actually happening, though, is that the more intense pigments in the shrimp’s shell are decomposing with heat, while the compound responsible for the red color is more stable. That red molecule is called astaxanthin, and it’s not only found in shrimp shells but is also the reason that salmon meat is red.