Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Fluorine
The lightest of the halogens, fluorine is the one that was isolated last, although its compounds have been used for centuries. Because of its incredibly high reactivity, elemental fluorine never occurs freely in nature and proved to be extremely difficult to isolate. Indeed, although hydrofluoric acid, HF, was known in the nineteenth century, attempts to oxidize the fluorine to the free element from either HF or any fluoride-bearing ore proved elusive, and in several cases had harmful consequences to those experimenting with it. There is more than one injury and fatality associated with attempts throughout the nineteenth century to isolate the element. These individuals are today sometimes referred to as the fluorine martyrs.
Even today, elemental fluorine and hydrofluoric acid must be stored in plastic containers, as the materials etch glass, and thus would destroy glass containers. Despite this high reactivity, fluorine has become an extremely useful element, finding uses in applications as different as aluminum refining and the clothing industry. Several modern processes and products would not exist without the direct or indirect incorporation of fluorine, hydrofluoric acid, or some fluoride salt.
22.1 Isolation and production
Fluorine exists in the reduced form as fluoride in a wide variety of minerals. Three compounds, fluorite (CaF2), cryolite (Na3AlF6), and fluorapatite (Ca5(PO4)3F), are the main materials that are mined, from which fluorine or HF is extracted. Fluorite is so common that it can be found on all the inhabited continents (although only in small quantities in Australia) (Government of Southern Australia, 2014), but the fluorine or hydrofluoric acid extracted from it is now so crucial in several end applications that it is tracked by the United States Geological Survey Mineral Commodities Summary each year under the more common name “fluorspar” (USGS, 2014). The worldwide mine production of fluorite is shown in Figure 22.1. The United States is not listed, even though the figures are compiled from the USGS Mineral Commodities Summary, because of proprietary concerns (USGS, 2014).
The reaction chemistry that describes the isolation of elemental fluorine can be represented simply, as shown in Figure 22.2. While the reactions appear to be simple, there are numerous details that are essential to their successful functioning which are not easily expressed in a chemical equation. The reaction is an electrolysis, runs at low temperature (roughly 70—130 °C), uses platinum—iridium electrodes and surfaces (because the alloy is more resistant to corrosion than platinum alone), and originally utilized fluorite stoppers, again because they were resistant to the elemental product.
Fluorine was first isolated by Henri Moissan in 1886. Although there has recently been a published report of a chemical isolation of fluorine, industrial scale manufacturing of the element is not very much changed from that which gave Moissan his early success (he actually received the Nobel Prize in 1906 for this work).
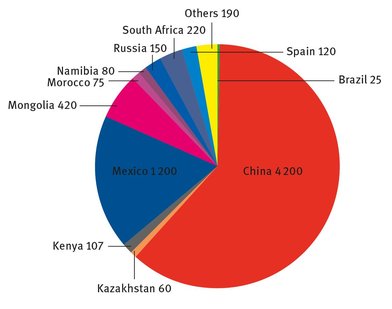
Fig. 22.1 : Worldwide fluorite production (in thousands of metric tons).
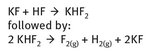
Fig. 22.2: Elemental fluorine isolation.
Elemental fluorine finds some industrial scale use, specifically in producing UF6 and SF6 for uranium enrichment and electrical insulation, respectively. The reaction chemistry can be represented as additions, shown in Figure 22.3.
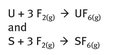
Fig. 22.3: UF6 and SF6 production.
Gaseous UF6 is used to concentrate U-235, which is the fissile isotope of uranium, but a small percentage of all uranium isotopes. Sulfur hexafluoride is used as a dielectric medium and is made on a scale of several thousand tons annually.
22.2 Hydrogen fluoride
In a large number of applications, elemental fluorine is not required to make a fluorinated product. Instead, hydrogen fluoride (HF) is used as the starting material. Hydrogen fluoride can be produced from the mineral fluorite — fluorspar — upon treatment with sulfuric acid. The reaction chemistry is straightforward, and is shown in Figure 22.4.
![]()
Fig. 22.4: Production of hydrofluoric acid.
The resulting HF is usually refined to two different levels of purity, each of which goes by a nonsystematic name. Acidspar is roughly 97% purity HF, and it is used for the production of fluorinated carbon-based materials, for the production of synthetic cryolite needed for aluminum refining, or for steel production. A small amount is refined to fluorine gas and combined with uranium for isotopic uranium enrichment. What is called metspar varies in purity from approximately 65—85%, and is used, with rare exception, for the smelting of iron. The two grades are produced in roughly equal amounts annually.
22.3 Cryolite
While cryolite can be a minable mineral, it is now rare; and the mine at Ivigtut, Greenland closed in 1987 because the ore was depleted beyond the point where extraction was profitable. Thus, synthetic cryolite has replaced mined ore as the source of the cryolite used in aluminum smelting. This can be made to high purity, and thus does not need the purification treatment that mined ores often do. The reaction chemistry illustrating the production of synthetic cryolite can be shown in Figure 22.5.
![]()
Fig. 22.5: Cryolite synthesis.
This leads to a large use of sodium hydroxide, which is itself one of the largest production inorganic chemicals in the world. The reaction also requires a significant outlay of electrical energy. It requires at least 10,000 A and 4.5 V for the synthesis. A comparison is useful, when looking at cryolite, to see what commodities are required in aluminum production. What is required for 1 ton of aluminum is:
1. 1.89 tons of Al2O3
2. Roughly 0.45 tons of carbon anode material
3. 0.07 tons of Na3AlF6
4. 15,000 kWh of electricity
Clearly, while cryolite is required for aluminum production, electrical power becomes the major expense (Greenwood and Earnshaw, 1980; Alcoa, 2014).
22.4 Teflon
Numerous halogenated carbon-based molecules have been produced in the past century, with those known as the chlorofluorocarbons finding uses as refrigerant gases and several other applications. But Teflon, more properly polytetrafluoroethylene (PTFE), produced from tetrafluoroethylene in a standard olefin polymerization that also requires hydrofluoric acid, has become a polymer that has changed the world in several ways.
Tetrafluoroethylene, the starting material for Teflon, in turn is produced from chloroform, according to the reactions as shown in Figure 22.6.
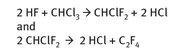
Fig. 22.6: Production of tetrafluoroethylene.
Clearly hydrofluoric acid is one of the reactants. Chloroform itself is made from the reaction of methane with chlorine gas, and thus the ultimate starting material for tetrafluoroethylene is natural gas.
The co-product hydrochloric acid must be captured for further use and is generally not discarded.
The story of the discovery and development of Teflon is one of the scientific successes of the 20th century, as well as one in which both serendipity and tragedy have a place. The discoverer of the material, Dr Roy Plunkett, found in 1938 while working for DuPont that a tank of tetrafluoroethylene he had planned to use in an experiment had polymerized in its tank. He was keen enough to isolate and identify the newly polymerized material, PTFE. The material, inadvertently produced at a laboratory scale, proved to be both highly corrosion resistant and rather expensive. The advent of the Second World War, and the need for corrosion resistant materials during the isolation of isotopically enriched uranium in the form of UF6 gas meant that the scale-up of PFTE became imperative for the war effort. Brigadier General Leslie Groves is credited for insisting that the isotopic enrichment tubing and equipment used in the Manhattan Project be changed from nickel metal, which did corrode to some extent in the purification process, to Teflon-lined tubing surfaces, which did not corrode in the presence of UF6 gas.
The reaction chemistry showing the production of Teflon from its monomer can be represented as shown in Figure 22.7. The polymerization is a free radical one, and the reaction can run at low temperatures (as evidenced by Plunkett’s original discovery).
The common perception is that Teflon is now used mainly as a coating on frying pans and other cookware, but the profile of uses for this per-fluorinated polymer is much wider than this. The DuPont website states:
Today, Teflon® coatings, and additives are used in paints, fabrics, carpets, home furnishings, clothing and so much more (DuPont, 2014).
While this is certainly a corporate perspective with a bias toward their products and materials, it does correctly indicate that Teflon finds extensive use in a variety of consumer products and materials, including fabrics.
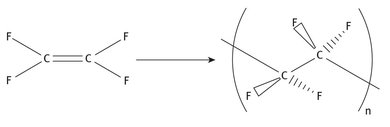
Fig. 22.7: Production of polytetrafluoroethylene.
22.5 Fluorinated fibers
Poly-tetrafluoroethylene has, in the past decades, become a valuable material in waterproof, outdoor clothing and in other fabrics for which the repelling of water is a major concern. The trade name Gortex® has become synonymous with such clothing in the minds of many (WL Gore & Associates, 2014). This fabric is made from PTFE that has been thermomechanically expanded and bonded to a base fabric.
The WL. Gore & Associates company continues to produce and market several other materials besides Gortex, which according to the corporate website includes: cables and cable assemblies, electronic and electrochemical materials, fabrics, fibers, filtration products, medical products, pharmaceutical processing, pump tubing, sealants, and venting products (WL Gore & Associates, 2014).
22.6 Dental fluoride and fluoridated water
In the past 60 years, the addition of small amounts of fluoride to drinking water in some countries (levels generally range from 0.5 to 1.0 mg/L) is believed to have helped reduce the number of cavities in the population. The advent of fluoridated toothpastes has also led to the decrease of cavities in the general population, especially in children. Ironically, the advent of fluoridated toothpastes coupled with this decline in cavities has actually led to calls for the removal of fluoride from drinking water, in part because of concerns that too much fluoride has been added in the water of some communities, and the belief that the fluoride supplied in toothpaste is more specifically targeted.
The debate about fluoride use in water and in communities can be quite heated, and at least one book, The Fluoride Deception, by Christopher Bryson, has been written about the subject, exploring it in detail (Bryson, 2004; International Society for Fluoride Research, 2014).
22.7 Recycling and reuse
The recycling of fluorine-containing materials is generally not something with which consumers are concerned, as they might be with such products as paper, aluminum cans, or glass and plastic bottles. Yet fluorides are often monitored closely and reused as much as possible, usually for economic reasons. For example, once cryolite has been synthesized, it is cost-effective to ensure its reuse in aluminum production for as long as possible.
Also, as one might expect, the accidental release of fluorine, hydrofluoric acid, and fluoride-containing materials can be deadly, and thus tight controls as well as continued reuse are common where these materials are concerned.
Bibliography
Alcoa. Website. (Accessed 30 May 2014, as: https://www.alcoa.com/global/en/about_alcoa/pdf/startswithdirt.pdf).
Bryson, C. The Fluoride Deception. Seven Stories Press, 2004, ISBN: 978-1-58322-526-4.
Recent report of chemical synthesis of fluorine.
DuPont website. (Accessed 24 February 2014, as: http://www2.dupont.com/Teflon/en_US/ ).
WL Gore & Associates. Website. (Accessed 30 May 2014, as: http://www.gore.com/en_xx/index.html).
Government of Southern Australia. Website. (accessed 24 February 2014, as: http://www.pir.sa.gov.au/minerals/geological_survey_of_sa/commodities/fluorite).
Greenwood, Earnshaw. Chemistry of the Elements, 2nd edn., Pergamon Press, 1980. ISBN 0 7506 3365 4.
International Society for Fluoride Research, Inc. Website. (Accessed 31 May 2014, as: http://www.fluorideresearch.org).
US Geological Survey, Mineral Commodity Summaries 2013. Website. (Accessed 26 January 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).