Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Chemical Foundations: Elements, Atoms, and Ions
Compounds That Contain Ions
Objective
· To learn how ions combine to form neutral compounds.
Chemists have good reasons to believe that many chemical compounds contain ions. For instance, consider some of the properties of common table salt, sodium chloride . It must be heated to about to melt and to almost to boil (compare to water, which boils at ). As a solid, salt will not conduct an electric current, but when melted it is a very good conductor. Pure water does not conduct electricity (does not allow an electric current to flow), but when salt is dissolved in water, the resulting solution readily conducts electricity (Fig. 4.18).
Figure 4.18.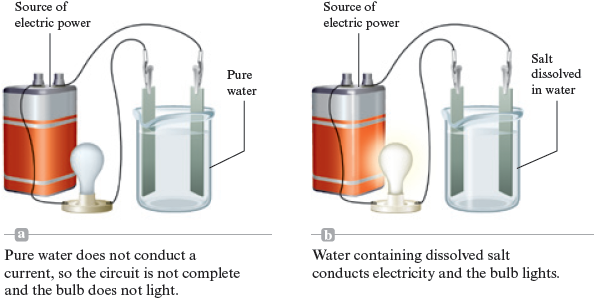
Chemists have come to realize that we can best explain these properties of sodium chloride by picturing it as containing ions and ions packed together as shown in Fig. 4.19. Because the positive and negative charges attract each other very strongly, it must be heated to a very high temperature before it melts.
Figure 4.19.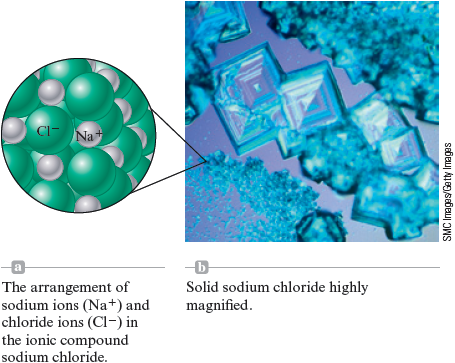
SMC Images/Getty Images
To explore further the significance of the electrical conductivity results, we need to discuss briefly the nature of electric currents. An electric current can travel along a metal wire because electrons are free to move through the wire; the moving electrons carry the current. In ionic substances the ions carry the current. Thus substances that contain ions can conduct an electric current only if the ions can move—the current travels by the movement of the charged ions. In solid the ions are tightly held and cannot move, but when the solid is melted and changed to a liquid, the structure is disrupted, and the ions can move. As a result, an electric current can travel through the melted salt.
The same reasoning applies to dissolved in water. When the solid dissolves, the ions are dispersed throughout the water and can move around in the water, allowing it to conduct a current.
Thus, we recognize substances that contain ions by their characteristic properties. They often have very high melting points, and they conduct an electric current when melted or when dissolved in water.
Many substances contain ions. In fact, whenever a compound forms between a metal and a nonmetal, it can be expected to contain ions. We call these substances ionic compounds .
Critical Thinking
· Thomson and Rutherford helped to show that atoms consist of three types of subatomic particles, two of which are charged. What if subatomic particles had no charge? How would this affect what you have learned?
One fact very important to remember is that a chemical compound must have a net charge of zero. This means that if a compound contains ions, then
1. Both positive ions (cations) and negative ions (anions) must be present.
2. The numbers of cations and anions must be such that the net charge is zero.
For example, note that the formula for sodium chloride is written , indicating one of each type of these elements. This makes sense because sodium chloride contains ions and ions. Each sodium ion has a charge, and each chloride ion has a charge, so they must occur in equal numbers to give a net charge of zero.

And for any ionic compound,
Consider an ionic compound that contains the ions and . What combination of these ions will give a net charge of zero? To balance the charge on , we will need two ions to give a net charge of zero.
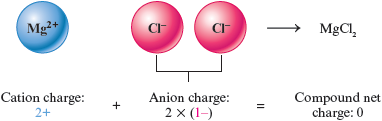
This means that the formula of the compound must be . Remember that subscripts are used to give the relative numbers of atoms (or ions).
Now consider an ionic compound that contains the ions and . What is the correct formula? These ions have charges of the same size (but opposite sign), so they must occur in equal numbers to give a net charge of zero. The formula of the compound is , because .
Similarly, the formula of a compound that contains the ions and is because three cations are needed to balance the charge of the anion.
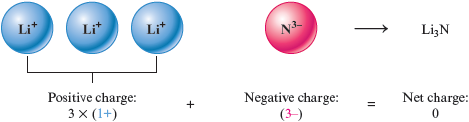
Interactive Example 4.6. Writing Formulas for Ionic Compounds
The pairs of ions contained in several ionic compounds follow. Give the formula for each compound.
a. and
b. and
c. and
Solution
a. has a charge, so two ions (each with the charge ) will be needed.
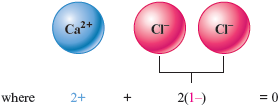
The formula is .
b. In this case , with its charge, requires two ions to produce a zero net charge.
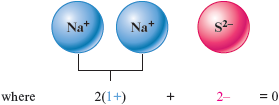
The formula is .
c. We have the ions (charge ) and (charge ). We must figure out how many of each are needed to balance exactly the positive and negative charges. Let’s try two and one .
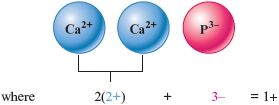
The resulting net charge is . This doesn’t work because the net charge is not zero. We can obtain the same total positive and total negative charges by having three ions and two ions.
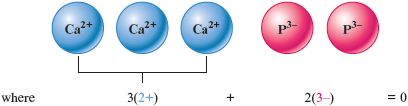
Thus the formula must be .
Self-Check: Exercise 4.6
· Give the formulas for the compounds that contain the following pairs of ions.
a. and
b. and
c. and
See Problems 4.83 and 4.84.