Liquid-State Physical Chemistry: Fundamentals, Modeling, and Applications (2013)
14. Some Special Topics: Reactions in Solutions
14.7. Ionic Solutions
In Chapter 11 we derived the activity coefficients for ionic solutions using the Debye–Hückel theory, which can be used immediately if the structure of the activated complex is known. For this structure, two extremes have been proposed: (i) a double-sphere model; and (ii) a single-sphere model. Each of these models will be discussed in the following sections (Figure 14.5).
Figure 14.5 The double-sphere and single-sphere models for the activated complex in an ionic reaction.
14.7.1 The Double-Sphere Model
For the double-sphere model consider the reaction A + B → M‡, where the reactants have charges zAe, zBe and (zA + zB)e, respectively. The activated complex is considered to be a double-sphere with r* the distance between A and B. The concentration of the activated complex is taken as proportional to the bulk concentration of A multiplied by the average concentration of B at a distance r*. The latter is given by
(14.57) ![]()
where cB is the bulk concentration of B, ψ is the mean electrostatic potential at a distance r* from A, and e is the unit charge. Hence, the concentration of activated complex is
(14.58) ![]()
with k′ the proportionality constant. Using the Debye–Hückel expression for ψ (Eq. 12.48), the expression becomes
(14.59) ![]()
where a is the mean distance of closest approach of A and B. The parameter κ is defined by the Debye length κ2 = 2NAe2Ic/ε0εrkT, where NA is Avogadro's constant and the ionic strength Ic is defined by ![]() . At high dilution, Eq. (14.59) becomes
. At high dilution, Eq. (14.59) becomes
(14.60) ![]()
and since the activity coefficient γ is equal to c0/c, we obtain
(14.61) ![]()
or, using Eq. (14.59) and (14.60)
(14.62) ![]()
The activity coefficient is with reference to the infinitely diluted solution, but we need this with respect to the gas phase. Here, we use α for the activity coefficient of the solution with respect to the gas phase, β for the activity coefficient of an infinitely diluted solution with respect to the gas phase, and γ for the activity coefficient of the solution with respect to the infinitely diluted solution. So
(14.63) ![]()
where the quantity involving the β-terms is given by
(14.64) ![]()
and the subscript “gas” indicates the ideal gas state with permittivity εr = 1. Applying the Debye–Hückel expression, this reads
(14.65) ![]()
Eliminating the activity coefficients α using Eq. (14.62), (14.63) and (14.65), the final result for the double-sphere model employing kAB = kAB,0(αAαB/α‡) is
(14.66) ![]()
14.7.2 The Single-Sphere Model
For the single-sphere model, one uses essentially the Born hydration model (see Section 12.2). The activated complex is considered to be a single-sphere of radius r, which is transferred from the gas phase to the liquid phase. For this process we showed in Chapter 12 that
(14.67) ![]()
and because for this process ΔG = kT lnβ (with β as the activity coefficient defined in the previous section), we obtain
(14.68) ![]()
Hence, it follows that
(14.69) ![]()
where r‡ is the radius of the (spherical) activated complex with charge (zA + zB)e. The activity coefficient γ is as given by the Debye–Hückel model (see Section 12.5)
(14.70) ![]()
and, if a mean distance a is assumed for the distance of closest approach of the ions, it follows that
(14.71) ![]()
Now, employing again kAB = kAB,0(αAαB/α‡) and introducing Eq. (14.69) and (14.71) in the α-terms of Eq. (14.63), the final result becomes
(14.72) ![]()
The expressions for the single-sphere and double-sphere models become identical when rA = rB = r‡. The expressions derived in this and the previous section can be tested in two ways: first, via the dependence on ionic strength while keeping the permittivity constant; and, second, via the dependence on permittivity of the solvent using data for infinite dilution, that is, extrapolated to zero ionic strength. This will be discussed in the next two sections.
Problem 14.4
Verify Eq. (14.66) and (14.72).
Problem 14.5
Discuss the difference in dependence on ionic radii between the single-sphere and double-sphere models for the rate constant. To that purpose, express rA, rB and r‡ for the single-sphere model in r*, and consider when the resulting expression differs significantly from the corresponding expression for the double-sphere model. Do you expect that experimental data on the rate constant can help to distinguish between the two models?
14.7.3 Influence of Ionic Strength
For both Eq. (14.66) and (14.72) the influence of the ionic strength is given by
(14.73) ![]()
where ![]() is the rate constant for infinite dilution. Inserting
is the rate constant for infinite dilution. Inserting ![]() = 2e2NAIc/εkT (Eq. 12.48) and neglecting κa in the term 1 + κa in comparison with unity, this expression reduces to
= 2e2NAIc/εkT (Eq. 12.48) and neglecting κa in the term 1 + κa in comparison with unity, this expression reduces to
(14.74) ![]()
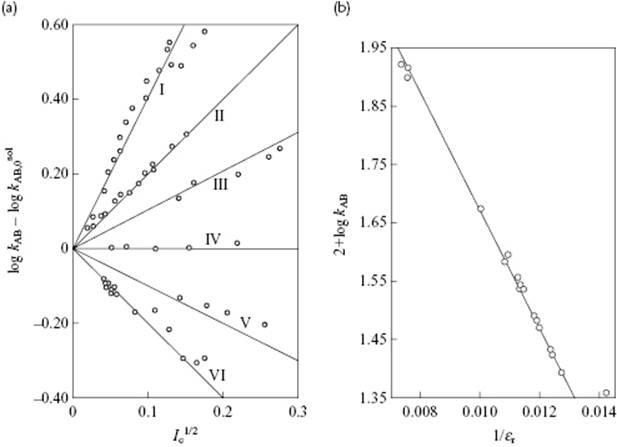
Introducing all numerical factors it appears that A = 1.02 (using mol l−1 for molarity) for aqueous solutions at 25 °C. In a plot of kAB versus ![]() , one should thus find a straight line with slope 1.02zAzB. The influence of the ionic strength for various reactions is shown in Figure 14.6a, where the experimental slopes nicely match the expected 1.02zAzB, including the zero slope if either zA or zB is zero.
, one should thus find a straight line with slope 1.02zAzB. The influence of the ionic strength for various reactions is shown in Figure 14.6a, where the experimental slopes nicely match the expected 1.02zAzB, including the zero slope if either zA or zB is zero.
Figure 14.6 Dependence of the specific rate constants on (a) ionic strength Ic and (b) permittivity εr. Panel (a): I. Co(NH3)5Br2+ + Hg2+, zAzB = +4; II. ![]() , zAzB = +2; III.
, zAzB = +2; III. ![]() , zAzB = +1; IV. CH3CO2C2H5 + OH−, zAzB = 0; V. H2O2 + H+ + Br−, zAzB = −1; VI. Co(NH3)5Br2+ + OH−, zAzB = −2. Panel (b): The reaction between bromoacetate and thiosulfate in various media. Data from Ref. [11].
, zAzB = +1; IV. CH3CO2C2H5 + OH−, zAzB = 0; V. H2O2 + H+ + Br−, zAzB = −1; VI. Co(NH3)5Br2+ + OH−, zAzB = −2. Panel (b): The reaction between bromoacetate and thiosulfate in various media. Data from Ref. [11].
Of course, deviations occur when the solution is too concentrated, that is, if Ic is too high. This occurs first, due to the further (mathematical) approximation made in the Debye–Hückel expression and, second, due to the approximate nature of the Debye–Hückel model itself. Moreover, for solvents with a low permittivity, the association of the ions may cause discrepancies, as has been shown for the reaction of sodium bromoacetate (Na+BrAc−) with sodium thiosulfate ![]() . At high dilutions BrAc− reacts with
. At high dilutions BrAc− reacts with ![]() so that zAzB = +2. However, at higher concentrations the complexes Na+···BrAc−,
so that zAzB = +2. However, at higher concentrations the complexes Na+···BrAc−, ![]() and
and ![]() are formed, and the effective value of zAzBchanges to zero. The repulsion between the negatively charged ions BrAc− and
are formed, and the effective value of zAzBchanges to zero. The repulsion between the negatively charged ions BrAc− and ![]() is decreased by the formation of complex ions, such that the specific reaction rate increases.
is decreased by the formation of complex ions, such that the specific reaction rate increases.
14.7.4 Influence of Permittivity
For a solution with ionic strength equal to zero, the terms in ![]() in Eq. (14.66) and (14.72) disappear and we are left with only the effect of the permittivity. Plotting lnkAB, extrapolated to zero ionic strength, versus 1/εr results for Eq. (14.66) in
in Eq. (14.66) and (14.72) disappear and we are left with only the effect of the permittivity. Plotting lnkAB, extrapolated to zero ionic strength, versus 1/εr results for Eq. (14.66) in
(14.75) ![]()
while for Eq. (14.72) one obtains
(14.76) ![]()
Both expressions give the same order of magnitude answer because the values of r*, rA, rB and r‡ are not too different. From Eq. (14.75) it is immediately clear that the slope has a negative sign if the ions are similarly charged, and positive if they are oppositely charged. This result can also be obtained from Eq. (14.76).
The influence of the permittivity on the rate constants extrapolated to zero ionic strength for the reaction between bromoacetate and thiosulfate in various media is shown in Figure 14.6b. Here, the line corresponds to a value of r* = 0.51 nm, but a good fit can also be obtained by using rA = 0.33 nm (bromoacetate), rB = 0.17 nm (thiosulfate) and r‡ = 0.50 nm (activated complex), respectively. The use of mole fractions instead of volume fractions (which are actually used in the single-sphere model) leads also to a good fit with r* = 0.56 nm. Yet, deviations can also occur, in particular for low-permittivity solvents.
Problem 14.6: The dissociation of AgNO3
The dissociation constants K for AgNO3 in H2O and C2H5OH are 1170 × 103 mol l−1 and 4.42 × 103 mol l−1, respectively. Estimate the value for K in CH3OH using the double-sphere model, and compare the result with the experimental value K = 20.5 × 103 mol l−1. Estimate the value for 2r* and compare the result with the sum of the Stokes radii for Ag+ and ![]() . Use, where necessary, data from Appendix E.
. Use, where necessary, data from Appendix E.