MCAT General Chemistry Review
Chapter 4: Compounds and Stoichiometry
4.5 Applications of Stoichiometry
Perhaps the most useful information to glean from a balanced reaction is the mole ratio of reactants consumed to products generated. One can also generate the mole ratio of one reactant to another or one product to another. All of these ratios can be generated using the stoichiometric coefficients. In the formation of water (2 H2 + O2 → 2 H2O), for example, one can determine that, for every one mole of hydrogen gas consumed, one mole of water can be produced; for every one mole of oxygen gas consumed, two moles of water can be produced. Furthermore, mole-to-mole, hydrogen gas is being consumed at a rate twice that of oxygen gas.
KEY CONCEPT
Stoichiometry, an application of dimensional analysis, is often simplified to a series of three fractions. These fractions demonstrate an underlying three-step process:
· Convert from the given units to moles
· Use the mole ratio
· Convert from moles to the desired units
Stoichiometry problems usually involve at least a few unit conversions, so take care when working through these types of problems to ensure that units cancel out appropriately to lead to the desired units of the answer choices. Pay close attention to the following problem, which demonstrates a clear and easy-to-follow method for keeping track of the numbers, calculations, and unit conversions.
Example:
How many grams of calcium chloride are needed to prepare 71.7 g of silver chloride according to the following equation?
CaCl2 (aq) + 2 AgNO3 (aq) → Ca(NO3)2 (aq) + 2 AgCl (s)
Solution:
Noting first that the equation is balanced, 1 mole of CaCl2 yields 2 moles of AgCl when it is reacted with 2 moles of AgNO3. The molar mass of CaCl2 is 111.1 g, and the molar mass of AgCl is 143.4 g. Our given quantity is 71.7 g AgCl.

Thus, about 27.8 g CaCl2 are needed to produce 71.7 g AgCl.
MCAT EXPERTISE
Common conversions used in stoichiometry include:
· 1 mole of any ideal gas at STP = 22.4 L
· 1 mole of any substance = 6.022 × 1023 particles (Avogadro’s number)
· 1 mole of any substance = its molar mass (from the Periodic Table)
LIMITING REAGENT
Rarely are reactants added in the exact stoichiometric proportions shown in the balanced equation of a reaction. As a result, in most reactions, one reactant will be used up or consumed first. This reactant is known as the limiting reagent (or reactant) because it limits the amount of product that can be formed in the reaction. The reactants that remain after all the limiting reagent is used up are called excess reagents (or reactants).
MCAT EXPERTISE
When the quantities of two reactants are given on the MCAT, expect to have to figure out which is the limiting reagent.
Figure 4.8 shows a reaction vessel that has significant amounts of reactants A and B, which react in equal amounts to produce product C. On the left, before the reaction, there is more reactant A than B. After the reaction is over, there is more product C but there is reactant A left over. Thus, reactant A is considered in excess, and reactant B is considered limiting.
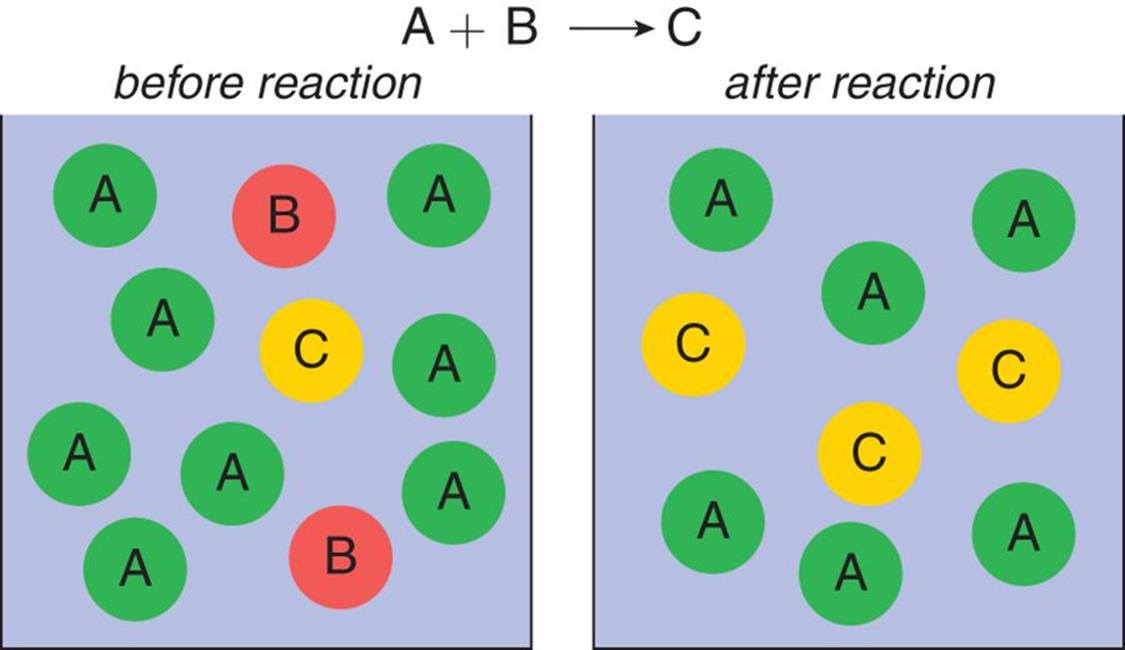 Figure 4.8. Reaction with a Limiting Reagent A is considered an excess reagent; B is the limiting reagent.
Figure 4.8. Reaction with a Limiting Reagent A is considered an excess reagent; B is the limiting reagent.
For problems involving the determination of the limiting reagent, keep in mind two principles:
1. All comparisons of reactants must be done in units of moles. Gram-to-gram comparisons will be useless and may even be misleading.
2. It is not the absolute mole quantities of the reactants that determine which reactant is the limiting reagent. Rather, the rate at which the reactants are consumed (the stoichiometric ratios of the reactants), combined with the absolute mole quantities determines which reactant is the limiting reagent.
Example:
If 27.9 g of Fe react with 24.1 g of S to produce FeS, what would be the limiting reagent? How many grams of excess reagent would be present in the vessel at the end of the reaction?
The balanced equation is Fe + S → FeS.
Solution:
First, determine the number of moles for each reactant.
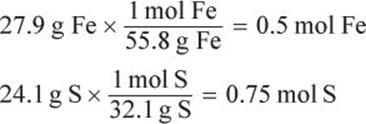
Because 1 mole of Fe is needed to react with 1 mole of S and there are 0.5 moles Fe for the given 0.75 moles S, the limiting reagent is Fe. Thus, 0.5 moles of Fe will react with 0.5 moles of S, leaving an excess of 0.25 moles of S in the vessel. The mass of the excess reagent will be:
![]()
YIELD
The yield of a reaction can refer to either the amount of product predicted (theoretical yield) or actually obtained (raw or actual yield) when a reaction is carried out. Theoretical yield is the maximum amount of product that can be generated as predicted from the balanced equation, assuming that all of the limiting reactant is consumed, no side reactions have occurred, and the entire product has been collected. Theoretical yield is rarely ever attained through the actual chemical reaction. Actual yield is the amount of product one actually obtains during the reaction. The ratio of the actual yield to the theoretical yield, multiplied by 100 percent, gives the percent yield:
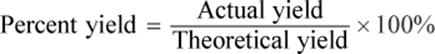
Equation 4.6
MCAT EXPERTISE
An experimentally based passage that involves a chemical reaction may include a pseudo-discrete question that involves finding the percent yield.
Example:
What is the percent yield for a reaction in which 27 g of Cu is produced by reacting 32.7 g of Zn in excess CuSO4 solution?
Solution:
The balanced equation is as follows:
Zn (s) + CuSO4 (aq) → Cu (s) + ZnSO4 (aq)
Calculate the theoretical yield for Cu.
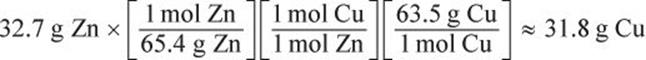
This 31.8 g represents the theoretical yield. Finally, determine the percent yield.

MCAT Concept Check 4.5:
Before you move on, assess your understanding of the material with these questions.
Questions 1–3 refer to the following unbalanced equation:
Na (s) + O2 (g) → Na2O (s)
1. Balance the chemical equation: ___ Na (s) + ___ O2 (g) → ___ Na2O (s)
2. If 50 g Na and 30 g O2 are provided, find the maximum number of moles of sodium oxide produced.
3. Identify the limiting reagent, and find the mass of the excess reagent left over once the reaction has run to completion.
4. Be(OH)2 is produced when water reacts with BeO. If one starts with 2 kg BeO in excess water, and produces 1.1 kg Be(OH)2, what is the percent yield of this reaction?