MCAT General Chemistry Review
Chapter 4: Compounds and Stoichiometry
Practice Questions
1. Which of the following best describes ionic compounds?
1. Ionic compounds are formed from molecules containing two or more atoms.
2. Ionic compounds are formed of charged particles and are measured by molecular weight.
3. Ionic compounds are formed of charged particles that share electrons equally.
4. Ionic compounds are three-dimensional arrays of charged particles.
2. Which of the following compounds has a formula weight between 74 and 75 grams per mole?
1. KCl
2. C4H10O
3. MgCl2
4. BF3
3. Which of the following is the gram equivalent weight of H2SO4 with respect to protons?
1. 49.1 g
2. 98.1 g
3. 147.1 g
4. 196.2 g
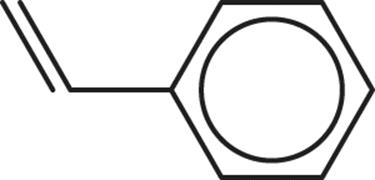
4. Which of the following molecules CANNOT be expressed by the empirical formula CH?
1. Benzene
2. Ethyne
3. 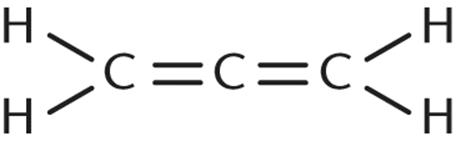
4. 
5. In which of the following compounds is the percent composition of carbon by mass closest to 62 percent?
1. Acetone
2. Ethanol
3. C3H8
4. Methanol
6. What is the most specific characterization of the reaction shown?
Ca(OH)2 (aq) + H2SO4 (aq) → CaSO4 (aq) + H2O (l)
1. Single-displacement
2. Neutralization
3. Double-displacement
4. Oxidation–reduction
7. In the reaction shown, if 39.05 g of Na2S are reacted with 113.3 g of AgNO3, how much of the excess reagent will be left over once the reaction has gone to completion?
Na2S + 2 AgNO3 → Ag2S + 2 NaNO3
1. 13.0 g Na2S
2. 26.0 g Na2S
3. 41.4 g AgNO3
4. 74.3 g AgNO3
8. Using a given mass of KClO3, how would one calculate the mass of oxygen produced in the following reaction, assuming it goes to completion?
2 KClO3 → 2 KCl + 3 O2
1. 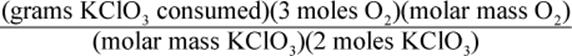
2. 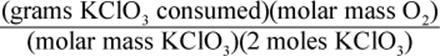
3. 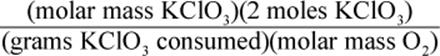
4. 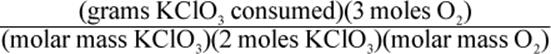
9. Aluminum metal can be used to remove tarnish from silver when the two solid metals are placed in water, according to the following reaction:
3 AgO + 2 Al → 3 Ag + Al2O3
This reaction is a:
1. double-displacement reaction.
2. single-displacement reaction.
3. oxidation–reduction reaction.
4. combination reaction.
1. II only
2. IV only
3. I and III only
4. II and III only
10.Which of the following types of reactions generally have the same number of reactants and products?
1. Double-displacement reactions
2. Single-displacement reactions
3. Combination reactions
1. I only
2. II only
3. I and II only
4. II and III only
11.A reaction that utilizes oxygen and hydrocarbons as reactants and that produces carbon dioxide and water as products is best characterized as:
1. single-displacement.
2. combustion.
3. metathesis.
4. decomposition.
12.In the process of photosynthesis, carbon dioxide and water combine with energy to form glucose and oxygen, according to the following equation:
![]()
What is the theoretical yield of glucose if 30 grams of water are reacted with excess carbon dioxide and energy, according to the equation above?
1. 30.0 g
2. 50.0 g
3. 300.1 g
4. 1801 g
13.In the following reaction:
Au2S3 (s) + H2 (g) → Au (s) + H2S (g)
If 2 moles of Au2S3 (s) is reacted with 5 moles of hydrogen gas, what is the limiting reagent?
1. Au2S3 (s)
2. H2 (g)
3. Au (s)
4. H2S (g)
14.Which of the following would make the strongest electrolytic solution?
1. A nonpolar covalent compound with significant solubility.
2. A ionic compound composed of one cation with +3 charge and three anions with −1 charge.
3. A polar covalent compound with a small dissociation constant.
4. An ionic compound composed of two cations with +1 charge and one anion with −2 charge.
15.What is the molecular formula of a compound with an empirical formula of B2H5 and a molar mass of ![]()
1. B2H5
2. B3H7
3. B4H10
4. B6H15
PRACTICE QUESTIONS