MCAT General Chemistry Review
Chapter 5: Chemical Kinetics
Practice Questions
1. In a third-order reaction involving two reactants and two products, doubling the concentration of the first reactant causes the rate to increase by a factor of 2. What will happen to the rate of this reaction if the concentration of the second reactant is cut in half?
1. It will increase by a factor of 2.
2. It will increase by a factor of 4.
3. It will decrease by a factor of 2.
4. It will decrease by a factor of 4.
2. In a certain equilibrium process, the activation energy of the forward reaction is greater than the activation energy of the reverse reaction. This reaction is:
1. endothermic.
2. exothermic.
3. spontaneous.
4. nonspontaneous.
3. A reactant in a second-order reaction at a certain temperature is increased by a factor of 4. By how much is the rate of the reaction altered?
1. It is unchanged.
2. It is increased by a factor of 4.
3. It is increased by a factor of 16.
4. It cannot be determined from the information given.
4. The concentrations of all reactants in a zero-order reaction are increased twofold. What is the new rate of the reaction?
1. It is unchanged.
2. It is decreased by a factor of 2.
3. It is increased by a factor of 2.
4. It cannot be determined from the information given.
5. Which of the following experimental methods should NEVER affect the rate of a reaction?
1. Placing an exothermic reaction in an ice bath.
2. Increasing the pressure of a reactant in a closed container.
3. Putting the reactants into an aqueous solution.
4. Removing the product of an irreversible reaction.
6. What would increasing the concentration of reactants accomplish in a solution containing a saturated catalyst?
1. It would increase the rate constant but not the reaction rate.
2. It would decrease the rate constant but increase the reaction rate.
3. It would increase the rate constant and increase the reaction rate.
4. The reaction rate would be unaffected.
7. A certain chemical reaction has the following rate law:
rate = k[NO2][Br2]
Which of the following statements necessarily describe(s) the kinetics of this reaction?
1. The reaction is second-order.
2. The amount of NO2 consumed is equal to the amount of Br2 consumed.
3. The rate will not be affected by the addition of a compound other than NO2 and Br2.
1. I only
2. I and II only
3. II and III only
4. I, II, and III
8. The following data shown in the table were collected for the combustion of the theoretical compound XH4:
XH4 + 2 O2 → XO2 + 2 H2O
|
Trial |
[XH4]initial (M) |
[O2]initial (M) |
|
|
1 |
0.6 |
0.6 |
12.4 |
|
2 |
0.6 |
2.4 |
49.9 |
|
3 |
1.2 |
2.4 |
198.3 |
What is the rate law for the reaction described here?
1. rate = k[XH4][O2]
2. rate = k[XH4][O2]2
3. rate = k[XH4]2[O2]
4. rate = k[XH4]2[O2]2
9. Which of the following best describes the purpose of a catalyst?
1. Catalysts are used up in the reaction, increasing reaction efficiency.
2. Catalysts increase the rate of the reaction by lowering the activation energy.
3. Catalysts alter the thermodynamics of the reaction to facilitate the formation of products or reactants.
4. Catalysts stabilize the transition state by bringing it to a higher energy.
10.If the rate law for a reaction is:
rate = k[A]0[B]2[C]1
What is the overall order of the reaction?
1. 0
2. 2
3. 3
4. 4
11.
1. For questions 11–13, consider the following energy diagram shown below:
2. 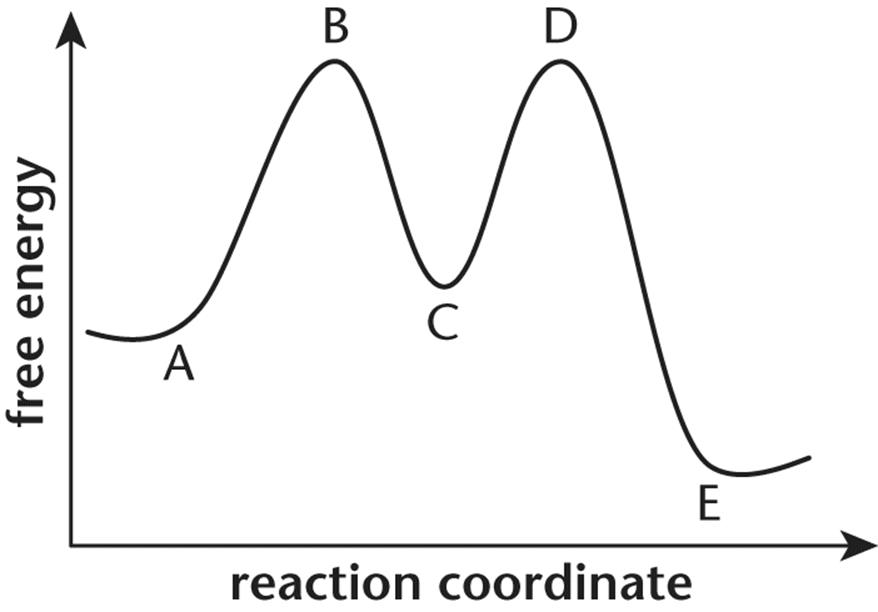
3. The overall reaction depicted by this energy diagramis:
1. endergonic, because point B is higher than point A.
2. endergonic, because point C is higher than point A.
3. exergonic, because point D is higher than point E.
4. exergonic, because point A is higher than point E.
4. Which process has the highest activation energy?
1. The first step of the forward reaction
2. The first step of the reverse reaction
3. The second step of the forward reaction
4. The second step of the reverse reaction
5. Point C in this reaction profile refers to the:
1. reactants.
2. products.
3. transition state.
4. intermediates.
12.The following system obeys second-order kinetics.
|
2 NO2 → NO3 + NO |
(slow) |
|
NO3 + CO → NO2 + CO2 |
(fast) |
13.What is the rate law for this reaction?
1. rate = k[NO2][CO]
2. rate = k[NO2]2[CO]
3. rate = k[NO2][NO3]
4. rate = k[NO2]2
13.The potential energy diagram shown represents four different reactions.
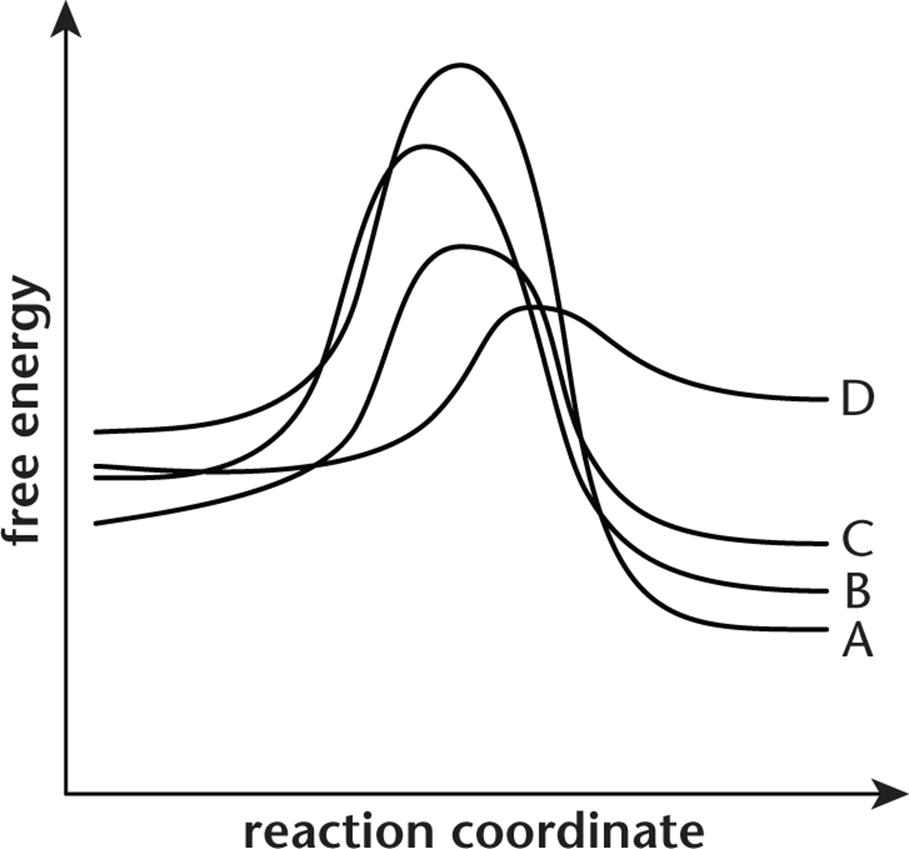
Assuming identical conditions, which of the reactions displayed on the energy diagram proceeds the fastest?
1. A
2. B
3. C
4. D
PRACTICE QUESTIONS