MCAT General Chemistry Review
Chapter 6: Equilibrium
Answers and Explanations
1. C
This scenario likely describes a situation in which a reaction has reached equilibrium very far to the right (with high product concentration and low reactant concentration). This reaction must be reversible because the reaction did not proceed all the way to the right. Any reaction in equilibrium has equal forward and reverse rates of reaction.
2. D
Recall that pure solids and liquids do not appear in the equilibrium expression; thus, this Keq has no denominator because the only reactant is a solid, cuprous sulfate. This could also be called Ksp because a solid is dissolving into ions in solution. The correct Keq should have [Cu+] squared because its stoichiometric coefficient is 2.
3. A
Carbon dioxide gas evolves and leaves the bottle, which decreases the total pressure of the reactants. Le Châtelier’s principle explains that a decrease in pressure shifts the equilibrium to increase the number of moles of gas present. This particular reaction will shift to the left, which in turn will decrease the amount of carbonic acid and increase the amount of carbon dioxide and water. Oxygen and nitrogen are not highly reactive and are unlikely to combine spontaneously with carbon dioxide or carbonic acid, as in choices (C) and (D).
4. A
Recall that equilibrium constants are either based on concentrations (Kc) or partial pressures (Kp). In this case, because all species are in the gas phase, we are using Kp—eliminating choices (B) and (D). When water is in the liquid phase, it does not appear in equilibrium expressions, as in choice (C). Here, however, water is in the gaseous phase and thus should appear in the equilibrium expression.
5. A
The larger the value of Keq (whether Kc or Kp), the larger the ratio of products to reactants. Therefore, if Kc ≫ 1, there are significantly larger concentrations of products than reactants at equilibrium. Even with a large Keq, the reaction will ultimately reach equilibrium far toward the products side and is therefore reversible, eliminating choice (D).
6. B
Adding sodium acetate increases the number of acetate ions present. According to Le Châtelier’s principle, this change will push this reaction to the left, resulting in a decrease in the number of free H+ ions. Because pH is determined by the hydrogen ion concentration, a decrease in the number of free protons will increase the pH. An acid’s Ka (which is simply the Keq for acid dissociation) will remain constant under a given temperature and pressure, eliminating choices(C) and (D).
7. ABoth increasing the pressure of the container and decreasing the volume would favor the side with fewer moles of gas, which is the product side. This makes choices (B) and (C) incorrect. Choice (D) would not disturb the equilibrium—the significance of decreasing the volume of the container in most equilibria is that there is an increase in pressure; in this case, however, the pressure remains constant despite the change in volume.
8. C
An exothermic reaction produces heat. Decreasing the temperature favors product formation, resulting in an increase in the forward reaction rate with a concomitant decrease in the reverse reaction rate.
9. B
The equilibrium of a reaction can be changed by several factors. Adding or subtracting heat, choice (A), would shift the equilibrium based on the enthalpy change of the reaction. Increasing reactant concentrations would shift the equilibrium in the direction of the product, and the opposite would occur if reactant concentrations were decreased, eliminating choice (C). Changing the volume of a reactant would affect any reaction with gaseous reactants or products, eliminating choice (D). While adding or removing a catalyst would change the reaction rate, it would not change where the equilibrium lies.
10.A
The first step to answering this question is to write out the balanced equation for the reaction of H2 and N2 to produce NH3: N2 + 3 H2 ⇌ 2 NH3. This means that Kc is equal to 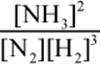 . Because the volume is 1 L, the amount of each gas (in moles) is equal to the value of the concentration of each gas (in M). If we start with 1 mole N2 and 3 moles H2, some amount x of N2 will react with 3x amount of H2 to form 2x amount of ammonia according to their stoichiometric coefficients. The amount of ammonia at equilibrium is given here as 0.05 M; thus, 2x= 0.05 and x = 0.025. Thus, the concentration of N2 at equilibrium is 1 – 0.025 = 0.975 M, and the concentrations of H2 at equilibrium is 3 – 3 × 0.025 = 2.925 M. Note that these changes are negligible because the answer choices differ by powers of ten. Thus, we can plug into theKeq expression to get
. Because the volume is 1 L, the amount of each gas (in moles) is equal to the value of the concentration of each gas (in M). If we start with 1 mole N2 and 3 moles H2, some amount x of N2 will react with 3x amount of H2 to form 2x amount of ammonia according to their stoichiometric coefficients. The amount of ammonia at equilibrium is given here as 0.05 M; thus, 2x= 0.05 and x = 0.025. Thus, the concentration of N2 at equilibrium is 1 – 0.025 = 0.975 M, and the concentrations of H2 at equilibrium is 3 – 3 × 0.025 = 2.925 M. Note that these changes are negligible because the answer choices differ by powers of ten. Thus, we can plug into theKeq expression to get  .
.
11.B
At extremely high temperatures, reactants or products may decompose, which will affect the equilibrium and potentially destroy the desired products. Choice (A) implies that reactions have limits, which is true; however, this does not make increasing temperature unfavorable.Choice (C) is false because increasing temperature would also increase pressure, assuming constant volume. Choice (D) is incorrect because it refers to properties of irreversible reactions which would not be involved in an equilibrium between products and reactants.
12.B
Statement I is false because the addition of a catalyst could increase the rate constants of both the forward and reverse reactions. Statement II is true because—for products to come into existence—reactants must be used up. Statement III is also true: all K values are temperature-dependent.
13.C
Ka is equal to the ratio of products to reactants, with each species raised to its stoichiometric coefficient. A compound with a Ka greater than 10–7 contains more H+ cations than HA– anions at equilibrium, which makes it an acid. This means that the compound in question is likely to react with a compound that is basic. Of the four answer choices, NH3 is the only base.
14.C
Reaction 2 is simply the reverse of reaction 1. This means that Keq for reaction 2 is the inverse of Keq of reaction 1, so the answer is (0.1)–1 = 10.
15.A
A negative ΔH value indicates an exothermic reaction, meaning that the forward reaction produces heat. Visualize this as follows:
A + B ⇌ C + D + heat
This means that removing heat by decreasing the temperature is similar to removing any other product of the reaction. To compensate for this loss, the reaction will shift to the right, causing an increase in the concentrations of C and D as well as a decrease in the concentrations of A and B.