MCAT General Chemistry Review
Chapter 7: Thermochemistry
7.4 Enthalpy
Most reactions in the lab occur under constant pressure (at 1 atm) in closed thermodynamic systems. To express heat changes at constant pressure, chemists use the term enthalpy (H). Enthalpy is a state function, so we can calculate the change in enthalpy (ΔH) for a system that has undergone a process—for example, a chemical reaction—by comparing the enthalpy of the final state to the enthalpy of the initial state, irrespective of the path taken. The change in enthalpy is equal to the heat transferred into or out of the system at constant pressure. To find the enthalpy change of a reaction, ΔHrxn, one must subtract the enthalpy of the reactants from the enthalpy of the products:
ΔHrxn = Hproducts – Hreactants
Equation 7.4
A positive ΔHrxn corresponds to an endothermic process, and a negative ΔHrxn corresponds to an exothermic process. It is not possible to measure enthalpy directly; only ΔH can be measured, and only for certain fast and spontaneous processes. Thus, several methods have been developed to calculate ΔH for any process.
STANDARD HEAT OF FORMATION
The standard enthalpy of formation of a compound, ΔH°f, is the enthalpy required to produce one mole of a compound from its elements in their standard states. Remember that standard state refers to the most stable physical state of an element or compound at 298 K and 1 atm. Note thatΔH°f of an element in its standard state, by definition, is zero. The ΔH°f values of most known substances are tabulated. You do not need to memorize these values because they will be provided for you.
STANDARD HEAT OF REACTION
The standard heat of a reaction, ΔH°rxn, is the enthalpy change accompanying a reaction being carried out under standard conditions. This can be calculated by taking the difference between the sum of the standard heats of formation for the products and the sum of the standard heats of formation of the reactants:
ΔH°rxn = Σ ΔH°f,products − Σ ΔH°f,reactants
Equation 7.5
HESS’S LAW
Enthalpy is a state function and is a property of the equilibrium state, so the pathway taken for a process is irrelevant to the change in enthalpy from one equilibrium state to another. As a consequence of this, Hess’s law states that enthalpy changes of reactions are additive. When thermochemical equations (chemical equations for which energy changes are known) are added to give the net equation for a reaction, the corresponding heats of reaction are also added to give the net heat of reaction, as shown in Figure 7.8.
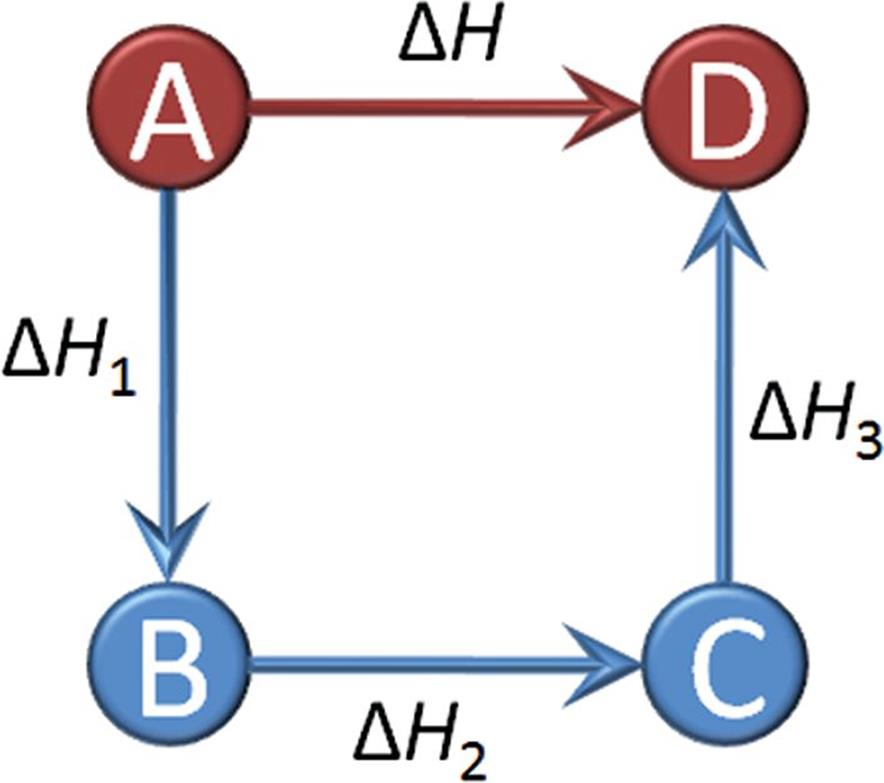 Figure 7.8. Illustration of Hess’s Law: Forming Product (D) from Reactant (A) Because enthalpy is a state function, ΔH = ΔH1 + ΔH2 + ΔH3
Figure 7.8. Illustration of Hess’s Law: Forming Product (D) from Reactant (A) Because enthalpy is a state function, ΔH = ΔH1 + ΔH2 + ΔH3
Hess’s law is embodied in the enthalpy equations we’ve already introduced. For example, we can describe any reaction as the result of breaking down the reactants into their component elements, then forming the products from these elements. The enthalpy change for the reverse of any reaction has the same magnitude, but the opposite sign, as the enthalpy change for the forward reaction. Therefore,
ΔHreactants → elements = –ΔHelements → reactants
The ΔHrxn can be written as:
ΔHrxn = ΔHreactants → elements + ΔHelements → products
which is another way of writing
ΔH°rxn = Σ ΔH°f,products − Σ ΔH°f,reactants
Consider the following phase change:
![]()
The enthalpy change for the phase change is called the heat of vaporization (ΔH°vap). As long as the initial and final states exist at standard conditions, the ΔH°vap will always equal the ΔH°vap, irrespective of the particular pathway that the process takes. For example, it’s possible that Br2 (l) could first decompose to Br atoms, which then recombine to form Br2 (g), rather than simply boiling from the liquid to gaseous state. However, because the net reaction is the same, the change in enthalpy will be the same.
KEY CONCEPT
State functions are always path independent.
Example:
Given the following thermochemical equations:
1. 
2. 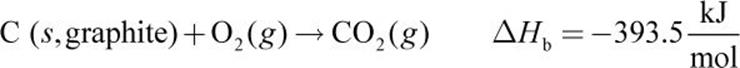
3. 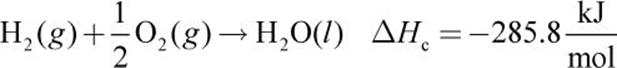
Calculate ΔH for this reaction:
1. C (s,graphite) + 2 H2 (g) → CH4 (g)
Solution:
Equations (a), (b), and (c) must be combined to obtain equation (d). Because equation (d) contains only C, H2, and CH4, we must eliminate O2, CO2, and H2O from the first three equations. Equation (a) is reversed to get CH4 on the product side (equation (e) below). Next, equation (b) is left as is (we will call this equation (f) for consistency below) and (c) is multiplied by 2 (equation (g) below). Then, (d) can be calculated from (e) + (f) + (g):
1. 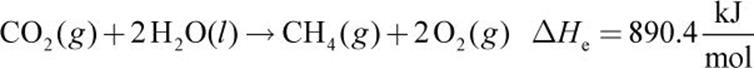
2. 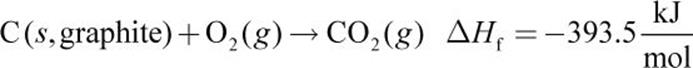
3. 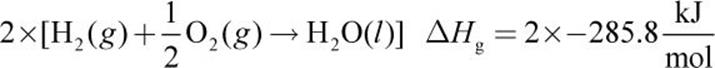
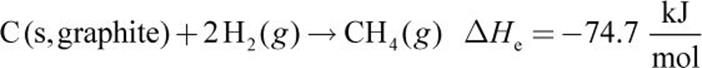
It is important to realize that Hess’s law applies to any state function, including entropy and Gibbs free energy.
MCAT EXPERTISE
When doing a problem like this on the MCAT, make sure to switch signs when you reverse the equation. Also, make sure to multiply by the correct stoichiometric coefficients when performing your calculations.
BOND DISSOCIATION ENERGY
Hess’s law can also be expressed in terms of bond enthalpies, also called bond dissociation energies. Bond dissociation energy is the average energy that is required to break a particular type of bond between atoms in the gas phase—remember, bond dissociation is an endothermic process. Bond dissociation energy is given in the units ![]() and is often given in tables on the MCAT in a format similar to Table 7.1.
and is often given in tables on the MCAT in a format similar to Table 7.1.
|
Bond |
|
|
O=O |
498 |
|
C–H |
415 |
|
H–H |
436 |
|
Table 7.1 Sample Bond Enthalpies |
|
Bond enthalpies are the averages of the bond energies for the same bond in many different compounds. For example, the C–H bond enthalpy 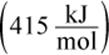 is averaged from measurements of the individual C–H bond enthalpies of thousands of different organic compounds. Note that bond formation, the opposite of bond breaking, has the same magnitude of energy but is negative rather than positive; that is, energy is released when bonds are formed. Remember that atoms generally form bonds to become more stable (often by completing an octet). Thus, it makes sense that bond formation is exothermic and bond dissociation is endothermic. The enthalpy change associated with a reaction is given by
is averaged from measurements of the individual C–H bond enthalpies of thousands of different organic compounds. Note that bond formation, the opposite of bond breaking, has the same magnitude of energy but is negative rather than positive; that is, energy is released when bonds are formed. Remember that atoms generally form bonds to become more stable (often by completing an octet). Thus, it makes sense that bond formation is exothermic and bond dissociation is endothermic. The enthalpy change associated with a reaction is given by
ΔH°rxn = Σ ΔHbonds broken − Σ ΔHbonds formed = total energy absorbed − total energy released
Equation 7.6
KEY CONCEPT
Because it takes energy to pull two atoms apart, bond breakage is always endothermic. The reverse process, bond formation, must always be exothermic.
Example:
Calculate the enthalpy change for the following reaction:
![]()
Bond dissociation energies of H–H and C–H bonds are ![]() and
and ![]() respectively. The ΔHf of C (g) is
respectively. The ΔHf of C (g) is ![]()
Solution:
CH4 is formed from free elements in their standard states (C in solid state and H2 in gaseous state). Thus, here ΔHrxn = ΔHf. The reaction can be written in three steps:
1. ![]()
2. ![]()
3. ![]()
with ΔHf = ΔH1 + 2 × ΔH2 + ΔH3
ΔH1 = ΔHf of ![]()
ΔH2 is the energy required to break the H–H bond of one mole of H2, so ΔH2 = bond energy of ![]()
ΔH3 is the energy released when 4 C–H bonds are formed. Because energy is released when bonds are formed, ΔH3 is negative.
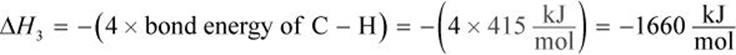
Therefore,
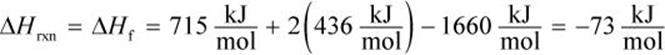
STANDARD HEAT OF COMBUSTION
As the name implies, the standard heat of combustion, ΔH°comb, is the enthalpy change associated with the combustion of a fuel. Because measurements of enthalpy change require a reaction to be spontaneous and fast, combustion reactions are the ideal processes for such measurements. Most combustion reactions presented on the MCAT occur in the presence of atmospheric oxygen, but keep in mind that there are other combustion reactions in which oxygen is not the oxidant. Diatomic fluorine, for example, can be used as an oxidant. In addition, hydrogen gas will combust with chlorine gas to form gaseous hydrochloric acid and, in the process, will evolve a large amount of heat and light as is characteristic of combustion reactions. The reactions listed in the CH4 (g) example shown earlier are combustion reactions with O2 (g) as the oxidant. Therefore, the enthalpy change listed for each of the three reactions is the ΔHcomb for each of the reactions.
The glycolytic pathway, described in Chapter 9 of MCAT Biochemistry Review, is also a combustion reaction that utilizes a fuel (glucose) mixed with an oxidant (oxygen) to produce carbon dioxide and water.
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
The heat of combustion for this reaction is found in a similar fashion to that of Hess’s Law. Given the numerous reactions and pathways involved we can determine the overall enthalpy of the reaction, as shown in Figure 7.9.
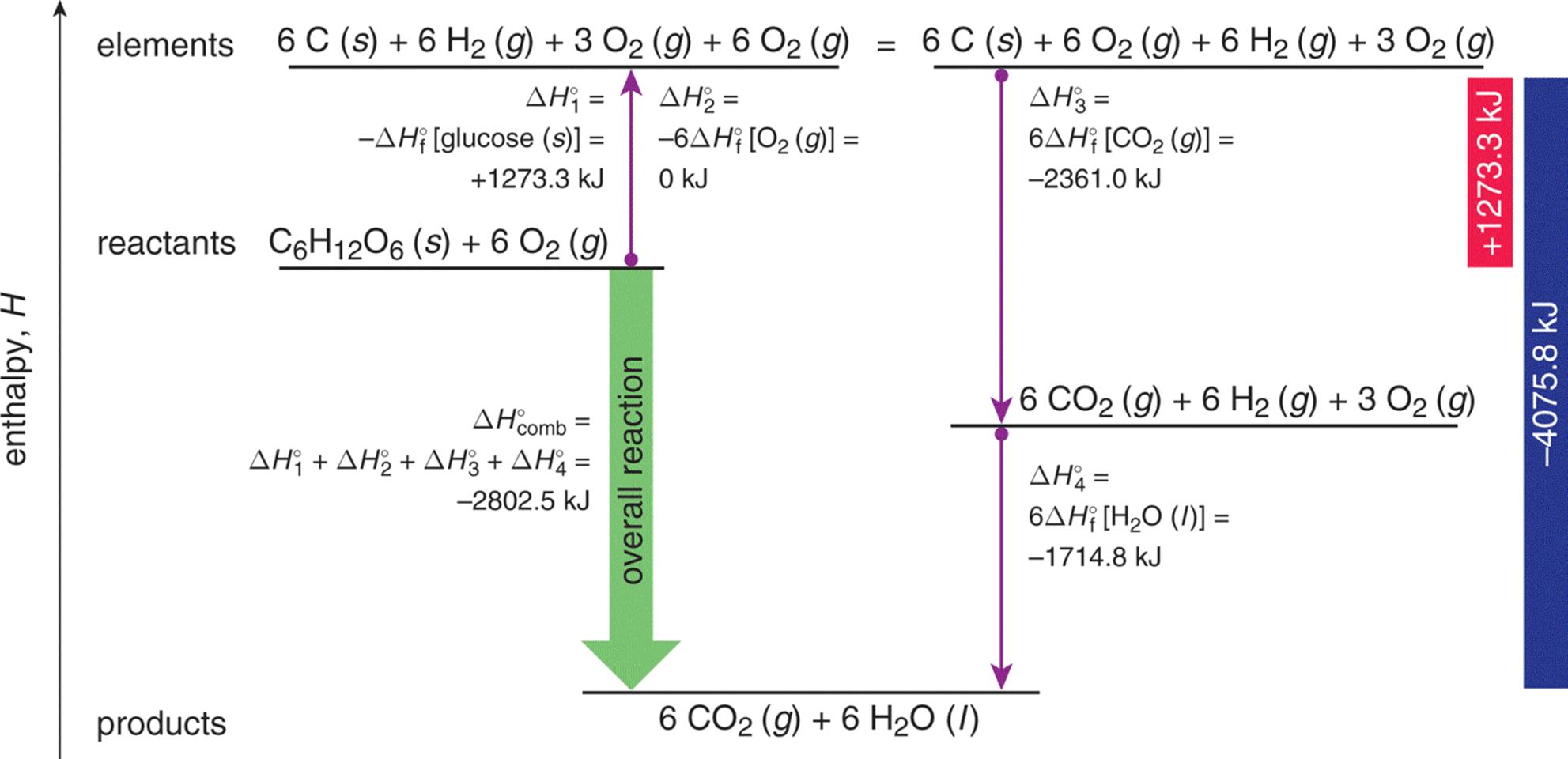 Figure 7.9. Determining the Enthalpy of Glycolysis
Figure 7.9. Determining the Enthalpy of Glycolysis
KEY CONCEPT
The larger the alkane reactant, the more numerous the combustion products.
MCAT Concept Check 7.4:
Before you move on, assess your understanding of the material with these questions.
1. Define endothermic and exothermic processes.
· Endothermic:
· Exothermic:
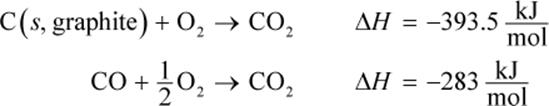
2. Given the following reactions, determine the enthalpy of C (s,graphite) + ![]()
3. What is the enthalpy of reaction for the reaction 2 H2O (g) → 2 H2 (g) + O2 (g), given the following bond enthalpies: ![]()
![]()
![]() and
and ![]()