MCAT General Chemistry Review
Chapter 7: Thermochemistry
Conclusion
We began our discussion of thermochemistry with a review of different ways in which we characterize systems (open, closed, and isolated) and processes (isothermal, adiabatic, isobaric, and isovolumetric). We then further classified systems according to their state functions—system properties such as pressure, density, temperature, volume, enthalpy, internal energy, Gibbs free energy, and entropy that describe the equilibrium state. We examined the equilibria that exist between the different phases and noted that the change in Gibbs free energy for each phase change in equilibrium is zero, as is the case for all equilibria. We defined enthalpy as the heat content of the system and the change in enthalpy as the change in heat content of the system as it moves from one equilibrium state to another. Enthalpy is defined as the energy found in the intermolecular interactions and bonds of the compounds in the system. We explored the various ways Hess’s law can be applied to calculate the total enthalpy change for a series of reactions. Moving on to entropy, we described this property as a measure of the degree to which energy in a system becomes spread out through a process. There is danger in thinking too literally about entropy as “disorder” because a system’s entropy may be increasing even if there is no observable change in the system’s macroscopic disorder (such as ice warming from –10°C to –5°C). Gibbs free energy combines the effects of temperature, enthalpy, and entropy, and the change in Gibbs free energy determines whether a process will be spontaneous or nonspontaneous. When the change in Gibbs free energy is negative, the process is spontaneous, but when the change in Gibbs free energy is positive, the process is nonspontaneous.
Many reactions in the body must be spontaneous in order for cells to function. While there are some nonspontaneous reactions in our body, we are able to couple them to thermodynamically favorable (exergonic) reactions that allow the cell to perform even more complex functions.
Concept Summary
Systems and Processes
· Systems are classified based on what is or is not exchanged with the surroundings.
o Isolated systems exchange neither matter nor energy with the environment.
o Closed systems can exchange energy but not matter with the environment.
o Open systems can exchange both energy and matter with the environment.
· Processes can be characterized based on a single constant property.
o Isothermal processes occur at a constant temperature.
o Adiabatic processes exchange no heat with the environment.
o Isobaric processes occur at a constant pressure.
o Isovolumetric (isochoric) processes occur at a constant volume.
States and State Functions
· State functions describe the physical properties of an equilibrium state; they are pathway independent and include pressure, density, temperature, volume, enthalpy, internal energy, Gibbs free energy, and entropy.
· Standard conditions are defined as 298 K, 1 atm, and 1 M concentrations.
· The standard state of an element is its most prevalent form under standard conditions; standard enthalpy, standard entropy, and standard free energy are all calculated under standard conditions.
· Phase changes exist at characteristic temperatures and pressures.
o Fusion (melting) and freezing (crystallization or solidification) occur at the boundary between the solid and the liquid phases.
o Vaporization (evaporation or boiling) and condensation occur at the boundary between the liquid and the gas phases.
o Sublimation and deposition occur at the boundary between the solid and gas phases.
o At temperatures above the critical point, the liquid and gas phases are indistinguishable.
o At the triple point, all three phases of matter exist in equilibrium.
· The phase diagram for a system graphs the phases and phase equilibria as a function of temperature and pressure.
Heat
· Temperature and heat are not the same thing.
o Temperature is a scaled measure of the average kinetic energy of a substance.
o Heat is the transfer of energy that results from differences of temperature between two substances.
· The heat content of a system undergoing heating, cooling, or phase changes is the sum of all the respective energy changes.
Enthalpy
· Enthalpy is a measure of the potential energy of a system found in intermolecular attractions and chemical bonds.
· Hess’s law states that the total change in potential energy of a system is equal to the changes of potential energies of the individual steps of the process.
· Enthalpy can also be calculated using heats of formation, heats of combustion, or bond dissociation energies.
Entropy
· Entropy, while often thought of as disorder, is a measure of the degree to which energy has been spread throughout a system or between a system and its surroundings.
o Entropy is a ratio of heat transferred per mole per unit kelvin.
o Entropy is maximized at equilibrium.
Gibbs Free Energy
· Gibbs free energy is derived from both enthalpy and entropy values for a given system.
· The change in Gibbs free energy determines whether a process is spontaneous or nonspontaneous.
o ΔG < 0: reaction proceeds in forward direction (spontaneous)
o ΔG = 0: reaction is in dynamic equilibrium
o ΔG > 0: reaction proceeds in reverse direction (nonspontaneous)
· Gibbs free energy depends on temperature; temperature-dependent processes change between spontaneous and nonspontaneous, depending on the temperature.
Answers to Concept Checks
· 7.1
1. The boundary between system and surroundings could be placed anywhere. Most commonly, the ice pack would be considered the chemical system using up energy, and the person (and the remainder of the universe) constitutes the surroundings that are providing the heat for the ice pack to function.
2.
· Isothermal: no change in temperature; ΔU = 0, Q = W
· Adiabatic: no heat exchange; Q = 0, ΔU = –W
· Isobaric: no change in pressure; line appears flat in a P–V graph
· Isovolumetric (isochoric): no change in volume; W = 0, ΔU = Q
· 7.2
1. Kinetics, equilibrium, and thermodynamics calculations use standard conditions, which are 25°C (298 K), 1 atm pressure, and 1 M concentrations.
2. State functions are properties of a system at equilibrium and are independent of the path taken to achieve the equilibrium; they may be dependent on one another. Process functions define the path between equilibrium states and include Q (heat) and W (work).
3. State functions include pressure (P), density (ρ), temperature (T), volume (V), enthalpy (H), internal energy (U), Gibbs free energy (G), and entropy (S).
4. The triple point is the combination of temperature and pressure at which all three phases are in equilibrium. The critical point is the temperature and pressure above which the liquid and gas phases are indistinguishable and the heat of vaporization is zero.
· 7.3
1. Temperature is an indirect measure of the thermal content of a system that looks at average kinetic energy of particles in a sample. Heat is the thermal energy transferred between objects as a result of differences in their temperatures.
2. Specific heat (c) is the energy required to raise the temperature of one gram of a substance by one degree Celsius. Heat capacity (mc) is the product of mass and specific heat and is the energy required to raise any given amount of a substance one degree Celsius.
3. A constant-pressure calorimeter (coffee cup calorimeter) is exposed to constant (atmospheric) pressure. As the reaction proceeds, the temperature of the contents is measured to determine the heat of the reaction. A constant-volume calorimeter (bomb calorimeter) is one in which heats of certain reactions (like combustion) can be measured indirectly by assessing temperature change in a water bath around the reaction vessel.
4. 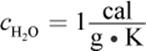
· 7.4
1. Endothermic reactions involve an increase in heat content of a system from the surroundings (ΔH > 0), while exothermic reactions involve a release of heat from a system (ΔH < 0).
2. Reverse the second equation, and then add the enthalpies:
![]()
3. Enthalpy of reaction = bonds broken – bonds formed. There are four O–H bonds broken, two H–H bonds formed, and one O=O bond formed. Therefore,
![]()
· 7.5
1. Solids have the lowest entropy, followed by liquids, with gases having the highest entropy.
2. Entropy increases as a system has more disorder or freedom of movement, and energy is dispersed in a spontaneous system. Entropy of the universe can never be decreased spontaneously.
3.
|
Reaction |
ΔS |
|
H2O (l) → H2O (s) |
Decrease (freezing) |
|
Dry ice sublimates into carbon dioxide |
Increase (sublimation) |
|
NaCl (s) → NaCl (aq) |
Increase (dissolution) |
|
N2 (g) + 3 H2 (g) → 2 NH3 (g) |
Decrease (fewer moles of gas) |
|
An ice pack is placed on a wound |
Increase (heat is transferred) |
· 7.6
1. At standard conditions:
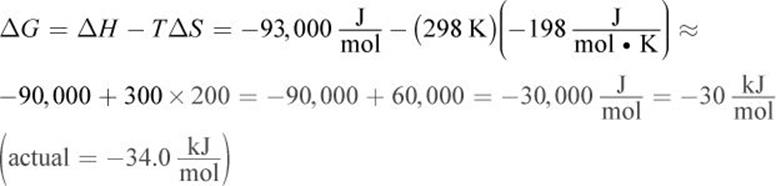
At 500 K:

2. The system is at equilibrium when
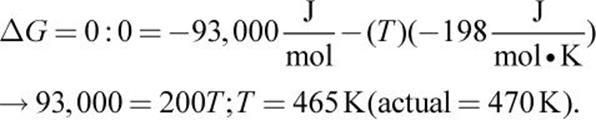
3. The value of Q would increase significantly, causing the system to shift left, forming more reactants until the system again reached equilibrium.
Equations to Remember
(7.1) First law of thermodynamics: ΔU = Q – W
(7.2) Heat transfer (no phase change): q = mcΔT
(7.3) Heat transfer (during phase change): q = mL
(7.4) Generalized enthalpy of reaction: ΔHrxn = Hproducts – Hreactants
(7.5) Standard enthalpy of reaction: ΔH°rxn = Σ ΔH°f,products − Σ ΔH°f,reactants
(7.6) Bond enthalpy:
ΔH°rxn = Σ ΔHbonds broken − Σ ΔH = total energy absorbed − total energy released
(7.7) Entropy: ![]()
(7.8) Second law of thermodynamics: ΔSuniverse = ΔSsystem + ΔSsurroundings > 0
(7.9) Standard entropy of reaction: ΔS°rxn = Σ ΔS°bonds broken − Σ ΔS°reactants
(7.10) Gibbs free energy: ΔG = ΔH – TΔS
(7.11) Standard Gibbs free energy of reaction: ΔG°rxn = Σ ΔG°f,products − Σ ΔG°f,reactants
(7.12) Standard Gibbs free energy from equilibrium constant: ΔG°rxn = –RT ln Keq
(7.13) Gibbs free energy from reaction quotient:
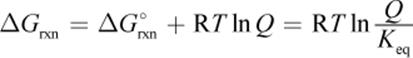
Shared Concepts
· General Chemistry Chapter 3
o Bonding and Chemical Interactions
· General Chemistry Chapter 4
o Compounds and Stoichiometry
· General Chemistry Chapter 5
o Chemical Kinetics
· General Chemistry Chapter 6
o Equilibrium
· Physics and Math Chapter 2
o Work and Energy
· Physics and Math Chapter 3
o Thermodynamics