MCAT General Chemistry Review
Chapter 1: Atomic Structure
Conclusion
Congratulations! You’ve made it through the first chapter! Now that we have covered topics related to the most fundamental unit of matter—the atom—you’re set to advance your understanding of the physical world in more complex ways. This chapter described the characteristics and behavior of the three subatomic particles: the proton, neutron, and electron. In addition, it compared and contrasted two models of the atom. The Bohr model is adequate for describing the structure of one-electron systems, such as the hydrogen atom or the helium ion, but fails to describe adequately the structure of more complex atoms. The quantum mechanical model theorizes that electrons are found not in discrete orbits, but in “clouds of probability,” or orbitals, by which we can predict the likelihood of finding electrons within given regions of space surrounding the nucleus. Both theories tell us that the energy levels available to electrons are not infinite but discrete and that the energy difference between levels is a precise amount called a quantum. The four quantum numbers completely describe the position and energy of any electron within a given atom. Finally, we learned two simple recall methods for the order in which electrons fill the shells and subshells of an atom and that the valence electrons are the reactive electrons in an atom. In the next chapter, we’ll take a look at how the elements are organized on the Periodic Table and will then turn our attention to their bonding behavior—based on valence electrons—in Chapter 3 of MCAT General Chemistry Review.
Concept Summary
Subatomic Particles
· A proton has a positive charge and mass around 1 amu; a neutron has no charge and mass around 1 amu; an electron has a negative charge and negligible mass.
· The nucleus contains the protons and neutrons, while the electrons move around the nucleus.
· The atomic number is the number of protons in a given element.
· The mass number is the sum of an element’s protons and neutrons.
Atomic Mass vs. Atomic Weight
· Atomic mass is essentially equal to the mass number, the sum of an element’s protons and neutrons.
o Isotopes are atoms of a given element (same atomic number) that have different mass numbers. They differ in number of neutrons.
o Most isotopes are identified by the element followed by the mass number (such as carbon-12, carbon-13, and carbon-14).
o The three isotopes of hydrogen go by different names: protium, deuterium, and tritium.
· Atomic weight is the weighted average of the naturally occurring isotopes of an element. The Periodic Table lists atomic weights, not atomic masses.
Rutherford, Planck, and Bohr
· Rutherford first postulated that the atom had a dense, positively charged nucleus that made up only a small fraction of the volume of the atom.
· In the Bohr model of the atom, a dense, positively charged nucleus is surrounded by electrons revolving around the nucleus in orbits with distinct energy levels.
· The energy difference between energy levels is called a quantum, first described by Planck.
o Quantization means that there is not an infinite range of energy levels available to an electron; electrons can exist only at certain energy levels. The energy of an electron increases the farther it is from the nucleus.
o The atomic absorption spectrum of an element is unique; for an electron to jump from a lower energy level to a higher one, it must absorb an amount of energy precisely equal to the energy difference between the two levels.
o When electrons return from the excited state to the ground state, they emit an amount of energy that is exactly equal to the energy difference between the two levels; every element has a characteristic atomic emission spectrum, and sometimes the electromagnetic energy emitted corresponds to a frequency in the visible light range.
Quantum Mechanical Model of Atoms
· The quantum mechanical model posits that electrons do not travel in defined orbits but rather are localized in orbitals; an orbital is a region of space around the nucleus defined by the probability of finding an electron in that region of space.
· The Heisenberg uncertainty principle states that it is impossible to know both an electron’s position and its momentum exactly at the same time.
· There are four quantum numbers; these numbers completely describe any electron in an atom.
o The principal quantum number, n, describes the average energy of a shell.
o The azimuthal quantum number, l, describes the subshells within a given principal energy level (s, p, d, and f).
o The magnetic quantum number, ml, specifies the particular orbital within a subshell where an electron is likely to be found at a given moment in time.
o The spin quantum number, ms, indicates the spin orientation 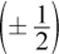 of an electron in an orbital.
of an electron in an orbital.
· The electron configuration uses spectroscopic notation (combining the n and l values as a number and letter, respectively) to designate the location of electrons.
o For example, 1s22s22p63s2 is the electron configuration for magnesium: a neutral magnesium atom has 12 electrons—two in the s subshell of the first energy level, two in the s subshell of the second energy level, six in the p subshell of the second energy level, and two in the ssubshell of the third energy level; the two electrons in the 3s subshell are the valence electrons for the magnesium atom.
· Electrons fill the principal energy levels and subshells according to increasing energy, which can be determined by the (n + l) rule.
· Electrons fill orbitals according to Hund’s rule, which states that subshells with multiple orbitals (p, d, and f) fill electrons so that every orbital in a subshell gets one electron before any of them gets a second.
o Paramagnetic materials have unpaired electrons that align with magnetic fields, attracting the material to a magnet.
o Diamagnetic materials have all paired electrons, which cannot easily be realigned; they are repelled by magnets.
· Valence electrons are those electrons in the outermost shell available for interaction (bonding) with other atoms.
o For the representative elements (those in Groups 1, 2, and 13−18), the valence electrons are found in s- and/or p-orbitals.
o For the transition elements, the valence electrons are found in s- and either d- or f-orbitals.
o Many atoms interact with other atoms to form bonds that complete an octet in the valence shell.
Answers to Concept Checks
· 1.1
1. Charge is determined by the number of electrons present. Atomic number is determined by the number of protons. Isotope is determined by the number of neutrons (while protons make up part of the mass number, it is the number of neutrons that explains the variability between isotopes).
2. 18O: 8p+, 10 n0, 8 e–. 18F: 9p+, 9 n0, 9 e–.
· 1.2
1. Atomic mass is (just slightly less than) the sum of the masses of protons and neutrons in a given atom of an element. Atoms of the same element with different mass numbers are isotopes of each other. The atomic weight is the weighted average of the naturally occurring isotopes of an element.
2. This ratio is an equivalent concept. It is therefore acceptable, as long as units can be cancelled in dimensional analysis.
3.
|
Isotope |
Protons |
Neutrons |
Electrons |
|
19O |
8 |
11 |
8 |
|
16O |
8 |
8 |
8 |
|
17O |
8 |
9 |
8 |
|
19F |
9 |
10 |
9 |
|
16F |
9 |
7 |
9 |
|
238U |
92 |
146 |
92 |
|
240F |
92 |
148 |
92 |
· 1.3
1. 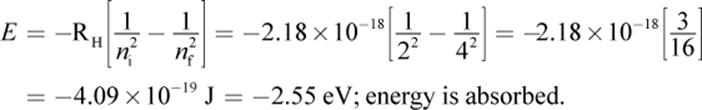
2. 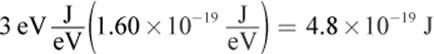

3. 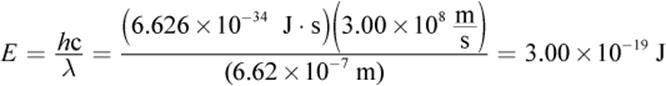
· 1.4
1.
|
n |
l |
Possible Elements |
|
2 |
1 |
2p: B, C, N, O, F, Ne |
|
3 |
0 |
3s: Na, Mg |
|
5 |
3 |
5f: Actinide series |
|
4 |
2 |
4d: Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd |
2. Both O and O2– have fully filled 1s- and 2s-orbitals. O has four electrons in the 2p subshell; two are paired, and the other two each have their own orbital. O2– has six electrons in the 2p subshell, all of which are paired in the three p-orbitals.
3. Both these molecules have unfilled valence electron shells with relatively few paired electrons; therefore, they are paramagnetic.
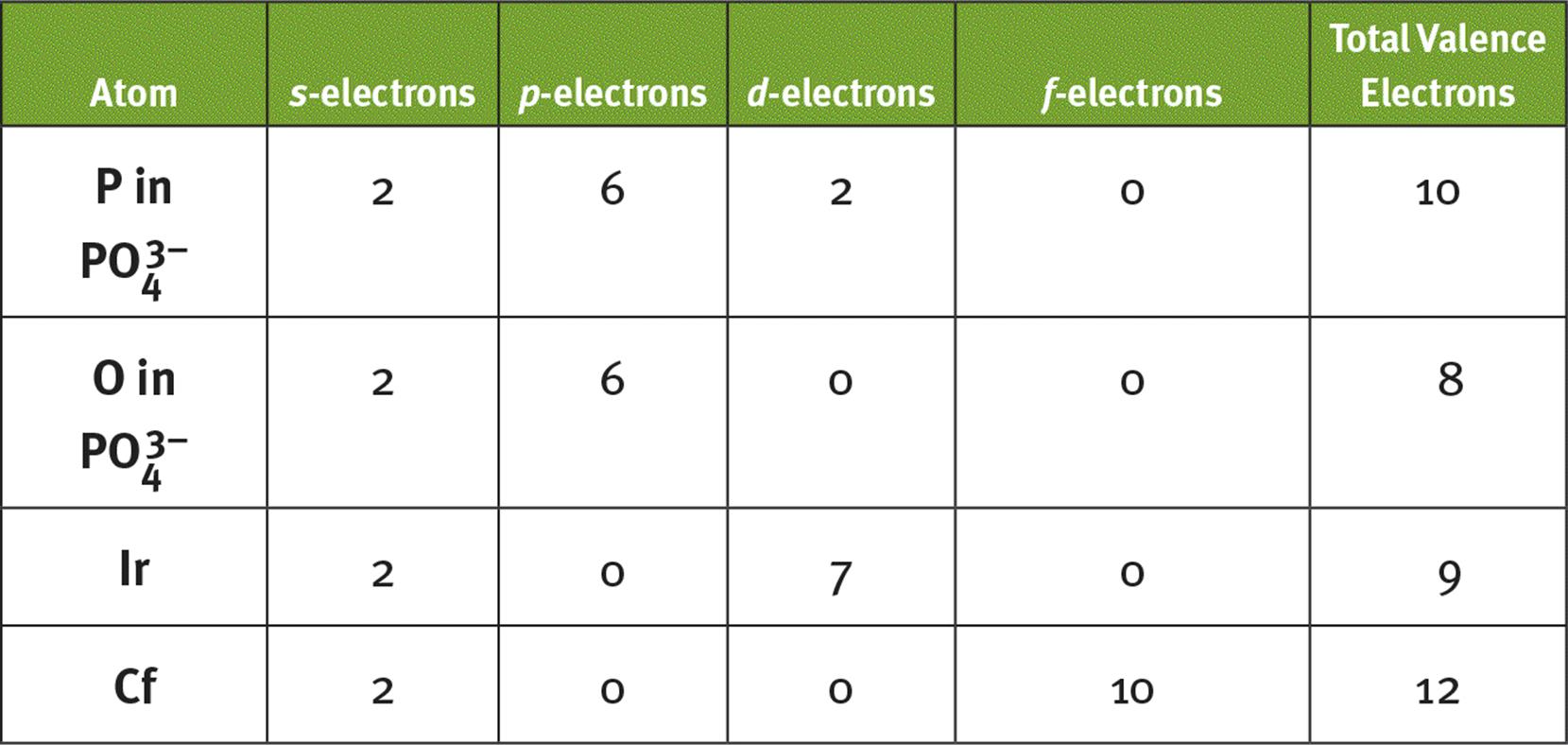
4. 
Equations to Remember
(1.1) Planck relation (frequency): E = hf
(1.2) Angular momentum of an electron (Bohr model): ![]()
(1.3) Energy of an electron (Bohr model): 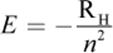
(1.4) Planck relation (wavelength): ![]()
(1.5) Energy of electron transition (Bohr model): 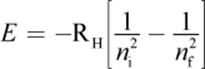
(1.6) Maximum number of electrons within a shell: 2n2
(1.7) Maximum number of electrons within a subshell: 4l + 2
Shared Concepts
· General Chemistry Chapter 2
o The Periodic Table
· General Chemistry Chapter 3
o Bonding and Chemical Interactions
· Organic Chemistry Chapter 3
o Bonding
· Physics and Math Chapter 2
o Work and Energy
· Physics and Math Chapter 8
o Light and Optics
· Physics and Math Chapter 9
o Atomic and Nuclear Phenomena