MCAT General Chemistry Review
Chapter 12: Electrochemistry
Conclusion
In this chapter, we covered the essential MCAT topic of electrochemistry. We reviewed the behavior of many different types of electrochemical cells. Galvanic cells rely on spontaneous oxidation–reduction reactions to produce current and supply energy. The concentration cell is a special type of galvanic cell for which the current is dependent on an ion concentration gradient rather than a difference in reduction potential between two chemically distinct electrodes. Electrolytic cells rely on external voltage sources to drive a nonspontaneous oxidation–reduction reaction called electrolysis. Finally, we considered the thermodynamics of the different cell types. Galvanic and concentration cells have positive electromotive forces (emf) and negative free energy changes, whereas electrolytic cells have negative electromotive forces and positive free energy changes.
In retrospect, the content you have learned in MCAT General Chemistry Review has numerous organic (biological) and inorganic applications. And as you prepare to be a physician, you must begin to understand and treat the individual as a sum of many intertwining systems and parts. Many body systems and parts rely on electrochemical cells: the heart is a self-innervating electrochemical cell, the neurons of the brain and spinal cord are rechargeable concentration cells, and every cell that contains mitochondria (all cells except erythrocytes) rely on the proton-motive force across the inner mitochondrial membrane to function. Our discussion here of inorganic systems has value through analogy to many biological systems.
Without further delay, we want to offer you our heartiest congratulations for completing this final chapter of MCAT General Chemistry Review. The hard work, time, and energy you have invested in a careful and thorough review of the topics covered within the pages of this book will pay off on Test Day. We hope that we have been successful in meeting our goals in writing this Kaplan MCAT Review series: to assess the general concepts and principles essential to correctly and efficiently answer the General Chemistry questions on the MCAT; to guide you in the development of critical thinking skills necessary for analyzing passages, question stems, and answer choices; and to provide holistic preparation for your Test Day experience. In addition to all of these, we aimed to relate the science to everyday life experiences and future experiences as a physician, demystify the concepts, and have some fun in the process. We are grateful for the opportunity to have been a part of your journey to success on the MCAT, and—beyond that—success in your medical education and future practice as the great physician you deserve to be!
Concept Summary
Electrochemical Cells
· An electrochemical cell describes any cell in which oxidation–reduction reactions take place. Certain characteristics are shared between all types of electrochemical cells.
o Electrodes are strips of metal or other conductive materials placed in an electrolyte solution.
o The anode is always the site of oxidation. It attracts anions.
o The cathode is always the site of reduction. It attracts cations.
o Electrons flow from the anode to the cathode.
o Current flows from the cathode to the anode.
· Cell diagrams are shorthand notation that represent the reactions taking place in an electrochemical cell.
o Cell diagrams are written from anode to cathode with electrolytes (the solution) in between.
o A vertical line represents a phase boundary, and a double vertical line represents a salt bridge or other physical boundary.
· Galvanic (voltaic) cells house spontaneous reactions (ΔG < 0) with a positive electromotive force.
· Electrolytic cells house nonspontaneous reactions (ΔG > 0) with a negative electromotive force. These nonspontaneous cells can be used to create useful products through electrolysis.
· Concentration cells are a specialized form of a galvanic cell in which both electrodes are made of the same material. Rather than a potential difference causing the movement of charge, it is the concentration gradient between the two solutions.
· The charge on an electrode is dependent on the type of electrochemical cell one is studying.
o For galvanic cells, the anode is negatively charged and the cathode is positively charged.
o For electrolytic cells, the anode is positively charged and the cathode is negatively charged.
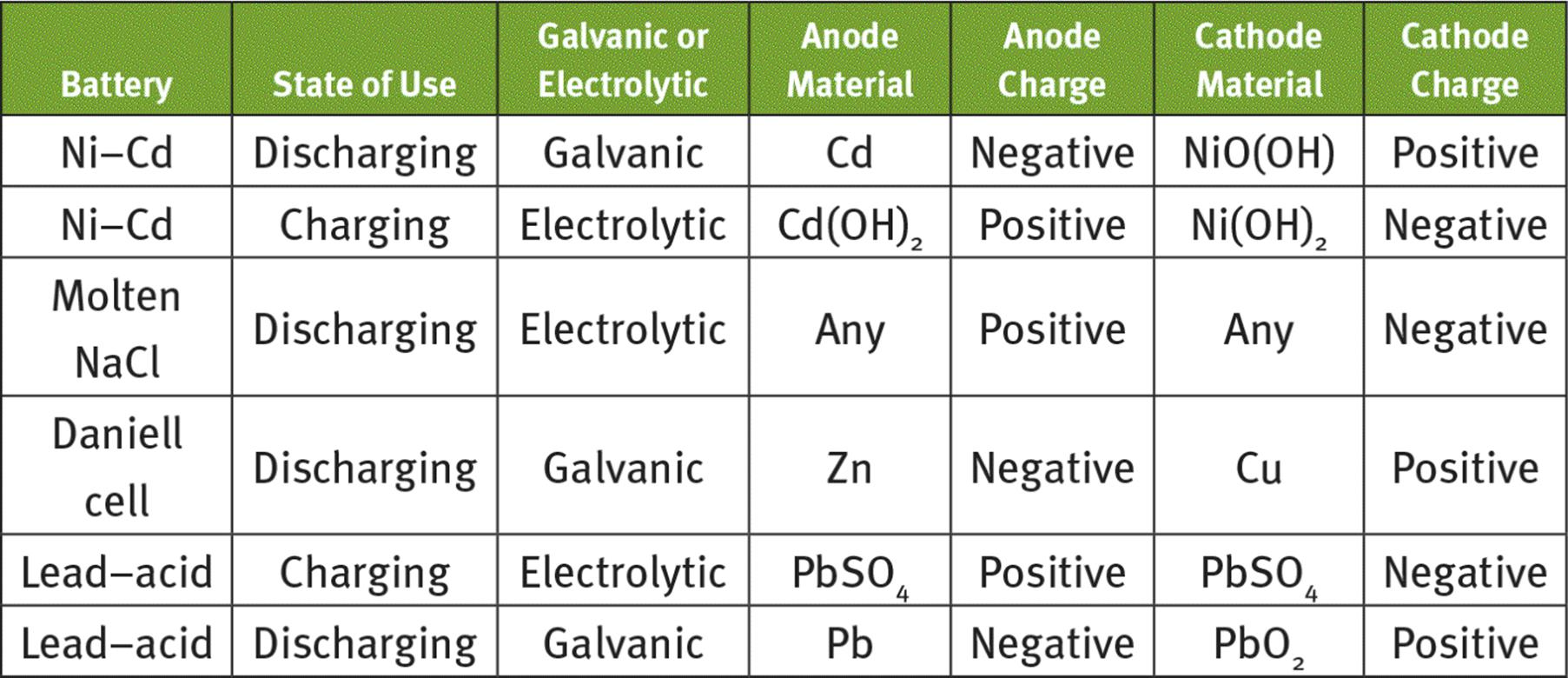
· Rechargeable batteries are electrochemical cells that can experience charging (electrolytic) and discharging (galvanic) states. Rechargeable batteries are often ranked by energy density—the amount of energy a cell can produce relative to the mass of battery material.
o Lead–acid batteries, when discharging, consist of a Pb anode and a PbO2 cathode in a concentrated sulfuric acid solution. When charging, the PbSO4-plated electrodes are dissociated to restore the original Pb and PbO2 electrodes and concentrate the electrolyte. These cells have a low energy density.
o Nickel–cadmium batteries (Ni–Cd), when discharging, consist of a Cd anode and a NiO(OH) cathode in a concentrated KOH solution. When charging, the Ni(OH)2- and Cd(OH)2-plated electrodes are dissociated to restore the original Cd and NiO(OH) electrodes and concentrate the electrolyte. These cells have a higher energy density than lead–acid batteries.
o Nickel–metal hydride (NiMH) batteries have more or less replaced Ni–Cd batteries because they have higher energy density, are more cost effective, and are significantly less toxic.
· Surge current is an above-average current transiently released at the beginning of the discharge phase; it wanes rapidly until a stable current is achieved.
Cell Potentials
· A reduction potential quantifies the tendency for a species to gain electrons and be reduced. The higher the reduction potential, the more a given species wants to be reduced.
o Standard reduction potentials (E°red) are calculated by comparison to the standard hydrogen electrode (SHE) under the standard conditions of 298 K, 1 atm pressure, and 1 M concentrations.
o The standard hydrogen electrode has a standard reduction potential of 0 V.
· Standard electromotive force (E°cell) is the difference in standard reduction potential between the two half-cells.
· For galvanic cells, the difference of the reduction potentials of the two half-reactions is positive; for electrolytic cells, the difference of the reduction potentials of the two half-reactions is negative.
Electromotive Force and Thermodynamics
· Electromotive force and change in free energy always have opposite signs.
o When E°cell is positive, ΔG° is negative. This is the case in galvanic cells.
o When E°cell is negative, ΔG° is positive. This is the case in electrolytic cells.
o When E°cell is 0, ΔG° is 0. This is the case in concentration cells.
· The Nernst equation describes the relationship between the concentration of species in a solution under nonstandard conditions and the electromotive force.
· There exists a relationship between the equilibrium constant (Keq) and E°cell.
o When Keq (the ratio of products’ concentrations at equilibrium over reactants’, raised to their stoichiometric coefficients) is greater than 1, E°cell is positive.
o When Keq is less than 1, E°cell is negative.
o When Keq is equal to 1, E°cell is 0.
Answers to Concept Checks
· 12.1
1. In a galvanic cell, the anode is the site of oxidation, has current flowing toward it, and has a (–) designation. The cathode has electrons flowing toward it and attracts cations.
2. In an electrolytic cell, the anode is the site of oxidation and has current flowing toward it. The cathode has electrons flowing toward it, has a (–) designation, and attracts cations.
3. Pb (s) | H2SO4 (4 M) || H2SO4 (4 M) | PbO2 (s)
4. Electrolytic cells are nonspontaneous and have a positive ΔG. Galvanic cells are spontaneous and have a negative ΔG; therefore, they have a positive Ecell.
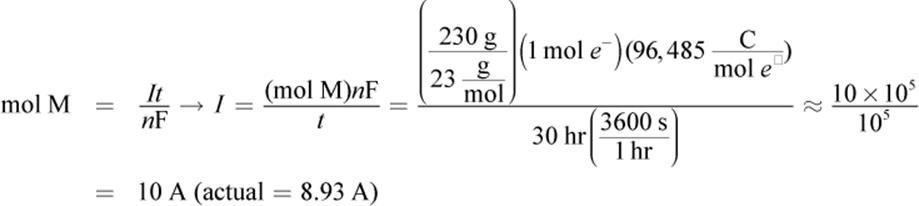
5. 
6. 
· 12.2
1. A sample is measured by setting up a cell relative to a standard hydrogen electrode, which is given a reduction potential of 0 V by convention.
2. A positive emf means the cell is spontaneous (galvanic); a negative emf means the cell is nonspontaneous (electrolytic).
3. The first cell is electrolytic because it has a negative emf. The second cell is galvanic because it has a positive emf.
4. The reduction potential of triiodide is higher than iron(III), so triiodide will be reduced and iron will be oxidized: 2 Fe + 3 I3− → 2 Fe3+ + 9 I− (E°cell = +0.57 V)
· 12.3
1.
|
Keq |
ΔG°: (+) or (−)? |
Reaction: Spontaneous or Nonspontaneous? |
E°cell: (+) or (−)? |
|
1.2 × 10–2 |
+ |
Nonspontaneous |
– |
|
2 × 102 |
– |
Spontaneous |
+ |
|
1 |
0 |
Not applicable—applies to any cell at equilibrium |
0 |
2. Remember that ΔG° = –RT ln Keq; if Keq < 1, ln Keq < 0, and ΔG° > 0. If Keq > 1, ln Keq > 0, and ΔG° < 0. If Keq = 1, ln Keq = 0, and ΔG° = 0.
2.
|
Q |
Keq |
Reaction Direction (Forward, Backward, or Equilibrium) |
Sign of Ecell |
|
10−3 |
10−2 |
Forward |
+ |
|
102 |
1.1 |
Backward |
– |
|
1 |
1 |
Equilibrium |
0 |
3. Note that these calculations do not assume standard conditions, unlike question 1.
Equations to Remember
(12.1) Moles of electrons transferred during reduction: Mn+ + n e– → M (s)
(12.2) Electrodeposition equation:
![]()
(12.3) Standard electromotive force of a cell: E°cell = E°red,cathode − E°red,anode
(12.4) Standard change in free energy from standard emf: ΔG° = −nFE°cell
(12.5) Nernst equation (full):
![]()
(12.6) Nernst equation (simplified):
![]()
(12.7) Reaction quotient:
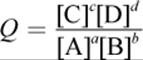
(12.8) Standard change in free energy from equilibrium constant: ΔG° = –RT ln Keq
(12.9) Free energy change (nonstandard conditions): ΔG = ΔG° + RT ln Q
Shared Concepts
· Biochemistry Chapter 3
o Nonenzymatic Protein Function and Protein Analysis
· Biochemistry Chapter 8
o Biological Membranes
· General Chemistry Chapter 7
o Thermochemistry
· General Chemistry Chapter 11
o Oxidation–Reduction Reactions
· Physics and Math Chapter 5
o Electrostatics and Magnetism
· Physics and Math Chapter 6
o Circuits