The MCAT Chemistry Book - Aryangat A. 2012
General Chemistry
Acid-Base Equilibrium
A. INTRODUCTION
In this chapter, we will explore different aspects involving acids and bases. We will start with the definitions, followed by ionization reactions, including various key concepts involving strong acids, strong bases, weak acids, weak bases, and buffers. Finally, we will discuss some of the important titration reactions.
B. DEFINITIONS OF ACIDS AND BASES
The Arrhenius Definition
According to Arrhenius, an acid is a substance that increases H+ ions in an aqueous solution. A base is a substance that increases OH— ions in an aqueous solution. The Arhhenius definitions explain the reactions involving acids that contain acidic hydrogens, and bases that contain basic hydroxyl groups (e.g., metal hydroxides). But the definitions are not able to explain the behavior of acids and bases of other types, such as in nonaqueous solutions.
The Bronsted-Lowry Definition
During the early twentieth century, scientists J.N. Bronsted and T.M. Lowry put forth their theories. According to them, an acid is a proton donor, and a base is a proton acceptor.
Consider the ionization reaction of hydrogen iodide. Since hydrogen iodide is a strong acid, it completely dissociates. In this ionization reaction, note that the water molecule acts as the base.
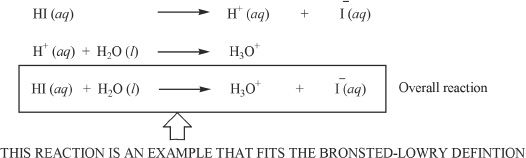
In this ionization reaction, obviously HI is the acid. HI transfers or donates its proton to water. Thus, water is the base in this reaction because it accepts the proton. The conjugate base of HI is I—.
C. THE LEWIS THEORY
The scientist G.N. Lewis put forward a more elaborate theory regarding acids and bases. According to him, a Lewis acid is a species that can accept a pair of electrons from another species. He defined Lewis base as any species that can donate a pair of electrons to another species. For a thorough understanding of this concept, let’s look at a typical Lewis acid-base reaction. The reaction between ammonia (NH3) and boron trichloride (BCl3) is shown:
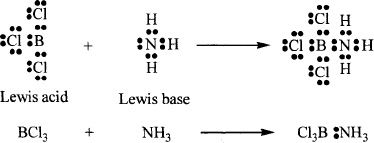
In this reaction, the electron acceptor is boron trichloride (Lewis acid) and the electron donor is ammonia (Lewis base). The new bond that is formed between the acid and the base is a coordinate covalent bond.
D. IONIZATION OF WATER
Usually pure water is considered to be a nonelectrolyte. Nevertheless, it actually can conduct small amounts of electricity. This is because of the self-ionization of water. Since the species that act as the acid and the base are one and the same, we can describe it as follows:
A proton is grabbed from one water molecule, and is accepted by another water molecule. The resulting products are hydronium and hydroxide ions.
![]()
The equilibrium expression (KW) is written as:
![]()
KW is more correctly called the ion-product constant for water, which is actually the equilibrium value of the ion product.
E. THE CONCEPT OF pH
In order to describe the acidity of a solution, scientists have devised the pH scale. The pH is defined as the negative log of the hydrogen-ion concentration.
![]()
Example 9-1
What is the pH of a solution having a 1 x 10—2 M hydrogen-ion concentration?
Solution:
The formula for pH is pH = — log [ H+ ]. Substituting in this formula,
pH = — log (1 x 10—2) = 2
The pH of this solution is 2.
We can also find pH if we know the hydroxide-ion concentration.
pOH = — log [OH—]
Since pH + pOH = 14, if we know the pOH, the pH can be easily calculated or vice versa.
Example 9-2
Find the pH of solution X, if it has a hydroxide-ion concentration of 1 x 10—8 M.
Solution:
Since the hydroxide-ion concentration is 1 x 10—8 M, the pOH is 8. Since we know that pH + pOH is 14,
pH = 14 — 8 = 6
The pH value of solution X is 6.
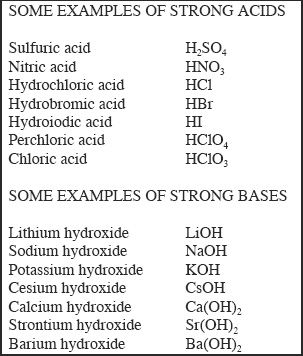
F. STRONG ACIDS AND BASES
Strong acids such as HNO3, HCl, and HI dissociate almost completely. If we were asked to predict the direction of such a dissociation reaction, we should say that the direction of reaction favors the dissociation of the acid. The dissociation of HI is shown below:
![]()
Since there is complete dissociation for every mole of HI present, equal number of hydronium ions are formed. You may be wondering whether protons can be supplied by the water molecules that are present. Usually this supply is negligible (the self-ionization of water is extremely low) compared to the concentration of the strong acid present. But this may not be always the case. Think about an instance when the proton contribution of water is significant in an acidic solution such as that of hydrogen iodide. When you have a strong acid solution which is very dilute, water does contribute protons significantly compared to that of the strong acid present.
Now let’s consider a strong base such as potassium hydroxide. Let’s say the solution is 1 x 10—3 M. Since KOH is a strong base, it dissociates completely. So we can confidently say that the concentration of hydroxide-ion formed is also 1 x 10—3 M. Just like the strong acid, we can ignore the possibility of hydroxide-ion formation from water, since the self-ionization of water is negligible. Since the OH— concentration is 1 x 10—3, the pOH is 3. From this, we can say that the pH of this solution is close to 11, since pH + pOH = 14.
A pH value below 7 is acidic, a pH value above 7 is basic, and a pH value 7 is neutral.
Table 9-1
G. WEAK ACIDS AND BASES
Weak acids and bases cannot dissociate completely. They undergo the same type of dissociation as that of strong acids and bases, but the extent of dissociation is very little compared to strong acid or strong base dissociations.
Acid-ionization Constant (Ka)
A typical way to represent the dissociation of a weak acid is shown below:
![]()
In this reaction, the protons from the weak acid are transferred to the water molecules. The acid-ionization constant (Ka) of this reaction is shown below:
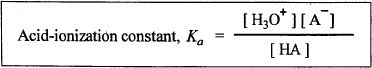
We encounter weak acids every day. An example is carbonated water that contains dissolved carbon dioxide, and is called carbonic acid. Fruits like lemons and oranges contain citric acid which is also a weak acid. The list goes on and on.
Percentage Ionization
Percentage ionization is the percentage expression of the degree of ionization. What is the degree of ionization of a weak acid? Degree of ionization is the fractional amount of the weak acid that gets ionized.
Example 9-3
Find the degree of ionization of 0.1 M solution of acetic acid (CH3COOH). Also find the pH of the solution.
(The acid-ionization constant of acetic acid is 1.8 x 10—5)
Solution:
![]()
We will logically dissect the events that lead to the dissociation. At first we do not have any H+ and CH3COO— ions. Let’s say we have a unit volume (a liter) of acetic acid and x M (mol/L) of it was ionized. We can represent the equation as follows:
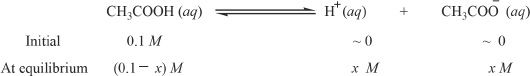
With the concentrations at equilibrium we can substitute the concentrations in the acid-ionization constant expression.
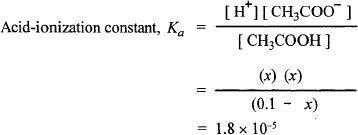
Even though this has to be solved using a quadratic equation, we can skip that elaborate process by making some chemically acceptable assumptions. Since the acid-ionization constant is very small, we can assume the same with the value of x. So the expression changes as follows. For the MCAT, you won’t be given problems that require extensive calculations. The problems will test mostly concepts, and when calculations are involved the numbers will usually be manageable ones.
![]()
Solving for x you will get roughly 1.34 x 10—3 = 0.00134 M.
![]()
The question also asks for pH of the solution.
pH = — log [ H+ ]
The hydrogen ion concentration is 0.00134 M. By substituting in the pH formula you should get the following result.
The pH of the solution is 2.87.
Base-ionization Constant (Kb)
Just like the acid-ionization constant, there is also the base-ionization constant. Consider the ionization of ammonia in water.
![]()
For the above reaction, we can write the base-ionization constant (Kb) for the above reaction as follows:
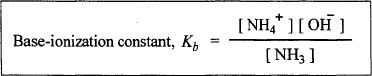
H. DISSOCIATION OF POLYPROTIC ACIDS
Some acids have two or more protons that can be released upon dissociation. Such acids are called polyprotic acids. For example, phosphoric acid (H3PO4) can lose up to three protons in aqueous solution.
Sulfuric acid is a polyprotic acid that can lose two protons in solution. The first ionization is complete because sulfuric acid is a strong acid.
![]()
The second proton comes off as depicted by the next equation. In this case, an equilibrium exists because hydrogen sulfate ion (HSO4—) is not as strong as H2SO4.
![]()
It is clear from this example that the second dissociation constant is always lower than the first dissociation constant. Thus, it is easier to remove a proton from an uncharged species H2SO4 than from a charged species (HSO4—).
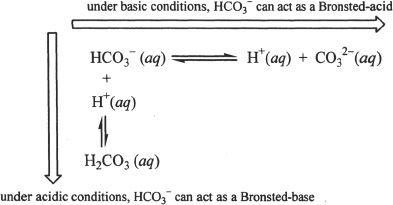
Based on the Bronsted-Lowry definition of acids and bases, an acid is a proton donor and base is a proton acceptor. A species (charged or uncharged) that can gain or lose a proton is called amphoteric or amphiprotic species. For example, (bicarbonate ion) HCO3— can donate a proton acting as an acid. The same species can accept a proton acting as a base. Hence, amphoteric species can act as an acid or a base depending on the surrounding conditions.
I. DIFFERENTIATING ACIDIC AND BASIC SALTS
Salt solutions can be acidic, neutral, or basic. Let’s consider a salt solution of NaF. NaF dissolves in water to form sodium and fluoride ions.
![]()
While the sodium ion is not reactive to water, the fluoride ion is reactive to water (fluoride is the conjugate base of a weak acid, HF). The fluoride ion can act as a base by accepting a proton from H2O by the process of hydrolysis. The reaction is shown below.
![]()
The increased presence of hydroxide ions (notice the formation of hydroxide ions as one of products of the hydrolysis reaction) makes the solution basic. This strategy can be used to predict whether a salt solution is acidic, basic, or neutral.
1. A salt of a strong base and a strong acid gives a neutral aqueous solution.
Eg: NaCl is a salt of a strong acid (HCl) and a strong base (NaOH).
2. A salt of a weak acid and a strong base gives a basic aqueous solution.
Eg: NaCN is a salt of a weak acid (HCN) and a strong base (NaOH).
3. A salt of a weak base and a strong acid gives an acidic aqueous solution.
Eg: Zn(NO)3 is a salt of a weak base (Zn(NO)2) and a strong base (HNO3).
J. BUFFERS
A buffer is a solution which can maintain a constant pH. If we add an acid or a base to a buffer solution, it can resist the change in pH to a certain extent. You have to remember that we are talking about small additions of acid or base, and such changes will not alter the buffer-pH much. But if we add a large quantity of acid or base to a buffer, obviously there will be significant change in its pH.
Buffers are conjugate acid-base mixtures. Buffer systems are very important for all types of organisms. The bicarbonate buffer system present in our body plays an important role in maintaining a reasonably constant pH. They are usually made of a weak acid with its conjugate base, or it can also be a mixture of a weak base with its conjugate acid. Consider the theory behind the working of a buffer system. Regardless of the type of buffer, both component species in a buffer system are in a state of equilibrium. Consider a weak acid-conjugate base buffer. A perfect example is a mixture of acetic acid and its salt. We can write the equation as follows:
![]()
Let’s say we are adding some acid into this buffer system. What happens to it? Well, as the acid is added, an increase in H3O+ is imperative. So the backward reaction is favored or in other words, the reaction proceeds to the left. This consumes the increased H3O+ and the system will try to attain equilibrium again. This process restores or maintains the pH, and that is exactly the function of a buffer system.
Consider what happens when we add a strong base (for example, KOH) to the same buffer system. The extra added OH— ions will consume the H3O+. As this happens, the reaction shifts to the right. So more and more acetic acid will ionize restoring the hydrogen ion concentration, and thereby the pH of the solution is maintained. Isn’t that amazing?
K. TITRATION CURVES OF ACIDS AND BASES
Acid-base titrations are reactions by which we can determine the amount of acid or base present in a solution. This is done by reacting the solution with a base or acid (of known concentration), and by measuring the volume of the known acid or base used up in the process. The reaction data is usually plotted with the volume of the substance (concentration known) in the x-axis, and the pH of the solution in the y-axis. The graph is called a titration curve.
Take a look at the first titration plot (Figure 9-1) given. You will see a steep region in the plot. The center of this steep region is called the equivalence point. The equivalence point denotes the point at which equivalent amounts of acid and base have reacted. To know the equivalence point, we usually add an indicator which will change its color close to the equivalence point. We can use the following relation to equate the amount of acid or base present in a solution against the volume of acid or base added whose concentration is known.

Indicators
An indicator is usually used to detect the equivalence point in an acid-base reaction or titration. The most common indicators used are weak organic acids or bases that change color in response to a change from acidic to basic medium or vice versa. The pH at which the color change occurs is characteristic of each indicator. For an acid-base reaction, the indicator is chosen based on the pH at which the equivalence point is expected to occur. Consider the hypothetical dissociation reaction of an indicator represented by HIn.
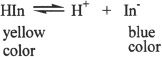
Let’s consider a scenario in which the indicator is in an acidic solution. Acidic solution means there is excess H+. So the equilibrium will shift to the left (LeChatelier’s principle), and the predominant species will be HIn making the indicator show yellow color. On the other hand, in a basic solution, the equilibrium will shift favoring the forward reaction and the predominant species will be In— (blue color). Because HIn (acid form) and In have different colors, this can indicate the equivalence point of acid-base reactions. You might be thinking that the dissociation of the indicator used itself might change or affect the pH of the tested solution. But this is negligible because only a tiny amount of the indicator is needed to show a visible color change.
Table 9-2
Some Common Indicators
Indicator |
Approx. pH Range of Color Change |
Color Change |
Thymol blue |
1.2-2.8 |
Red to Yellow |
Methyl Orange |
3.2-4.4 |
Red to Yellow |
Cresol Red |
7.1-8.9 |
Yellow to Red |
Thymol Blue |
8.0-9.7 |
Yellow to Blue |
Phenophthalein |
8.3-10.0 |
Colorless to Pink |
Alizarin Yellow |
10.0-12.0 |
Yellow to red |
Titration of a Strong Acid with a Strong Base
Consider the titration reaction involving HCl against NaOH. Let’s say that we have 50 ml of a 0.1 M solution of HCl, titrated with 0.1 M solution of NaOH. As we add NaOH into the solution of HCl, initially the pH increase will not be very drastic. The pH increases slowly. When certain amount of NaOH is added, the change in pH becomes more drastic and the plot becomes very steep, as indicated in the plot (Figure 9-1). At this steep region, the equivalence point is reached. Can you guess the pH at the equivalence point? If you guessed 7, you are right. At the equivalence point of this titration you have a salt which is neutral, and hence the pH is 7.
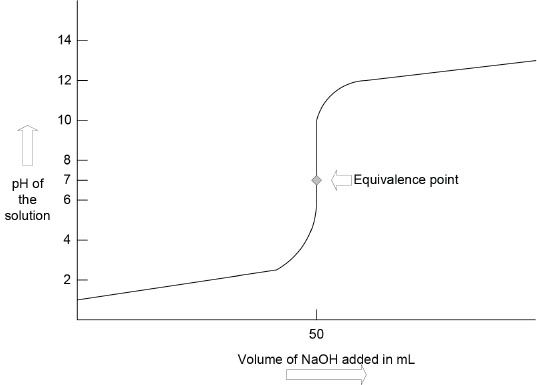
Figure 9-1
Titration of a Weak Acid with a Strong Base
The curve of a weak acid-strong base titration is different from that of the plot we learned for the strong acid-strong base titration. Unlike the previous curve, this plot will have a region which has buffering properties. Besides that, the equivalence point will be above the pH value 7. The general shape of the curve is shown in Figure 9-2.
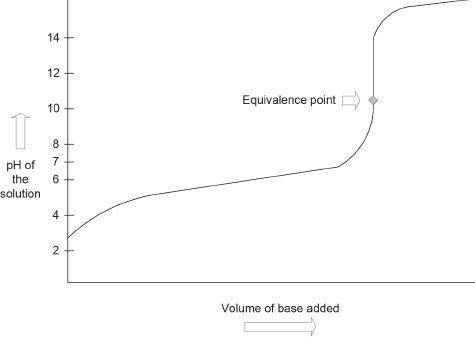
Figure 9-2
Titration of a Weak Base with a Strong Acid
The trend seen in the weak base-strong acid titration curve is somewhat similar to that of the weak acid-strong base curve. At first, as we add the acid, the pH slowly decreases. Then the decrease in pH becomes drastic, as you can see in Figure 9-3.
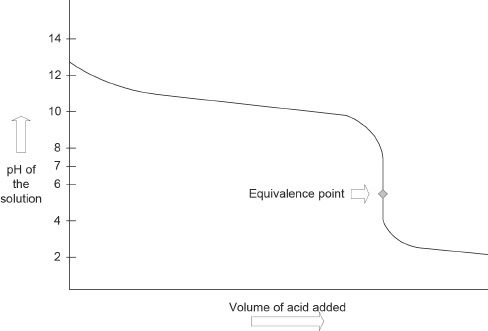
Figure 9-3
CHAPTER 9 PRACTICE QUESTIONS
1. If the pH of a solution is 2.5, what is the concentration of H3O+ in that solution?
A. 2.9 x 10—1
B. 3.2 x 10—3
C. 3.8 x 10—2
D. 6.3 x 10—5
2. NaCl solution is most likely:
A. acidic.
B. basic.
C. neutral.
D. cannot be predicted.
3. Which of the following is not a polyprotic acid?
A. H2SO4
B. H2PO4—
C. H3AsO4
D. None of the above
4. Based on the equation,
![]()
which of the following is not valid?
A. ![]()
B. ![]()
C. ![]()
D. None of the above are valid.
5. Which of the following species has the highest pKa value?
A. H2PO4—
B. H3PO4
C. HPO42—
D. All the above species have almost the same pKa values, since they all have the same type of phosphate anionic counterpart.
Questions 6-10 are based on the following passage.
Passage 1
Strong acids and bases ionize completely in aqueous solutions. Weak acids and bases ionize only partially in aqueous solutions. An acid-base reaction is shown below. A titration was done by adding a base to a known concentration and fixed volume of an acid. The reaction follows:
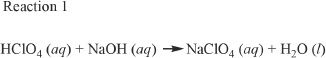
The dissociation of a weak acid can be represented as:
![]()
Chemists often use Henderson-Hasselbach equation to calculate the pH of solutions containing a weak acid of known pKa, provided that the other concentrations are known. Henderson-Hasselbach equation is:
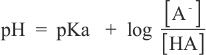
This equation is also used to calculate the buffering capability of solutions. It is roughly estimated that a given buffer is most likely to be effective at pH levels between (pKa + 1) and (pKa — 1).
6. The net ionic equation of Reaction 1 is:
A. ![]()
B. ![]()
C. ![]()
D. Perchloric acid is a acid and cannot ionize completely, so that net ionic equation cannot be written.
7. The titration curve of the acid-base reaction (Reaction 1) is best represented by:
A. 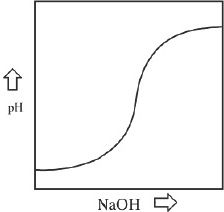
B. 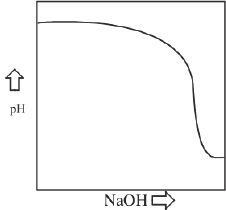
C. 
D. 
8. What are the base-to-acid ratios corresponding to the lower and upper limits of pH values which make the best buffer?
A. 10 and 1
B. 100 and 1
C. 0.01 and 10
D. 0.1 and 10
9. What is the pH of a 3.2 x 10—4 M solution of HCl?
A. 2.7
B. 3.5
C. 4
D. 4.4
10. The pH at which the base and the acid concentrations equal in a buffer system is called the:
A. equivalence point.
B. pKa.
C. neutralization point.
D. non-buffering zone.