Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
3. Microreactors Made of Glass and Silicon
Thomas Frank
This chapter describes the basic design technologies used to build a microreactor with glass and silicon. The technologies are very diverse, so only a general overview has been provided. The reader who wish to read about the concept in more detail can refer to the literature cited. The available technologies are diverse, so the reader may confine to details based on the requirements.
3.1. How Microreactors Are Constructed
In general, microreactors are systems of tiny channels in which a number of fluidizable substances are combined under specific physical conditions. The parameters of greatest importance are temperature, pressure, and dwell time, all of which are either set or altered by peripheral equipment such as pumps, heaters/coolers, and control systems.
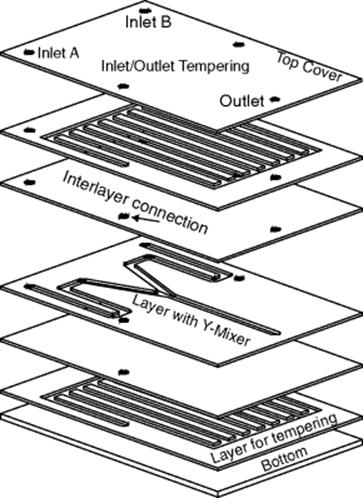
For the fabrication of such microreactors, there are a variety of materials available – metals, polymers, glass, ceramics, and semiconductors – and these are processed using microsystems engineering or conventional manufacturing methods. All the processes are geared to the creation of flat, planar parts, similar to the wafers made for microelectronics. These features tend to dictate the basic construction of microreactors. The systems of channels in the microreactors are formed of hermetically sealed layers bearing cannular structures. Modern bonding technology enables the individual layers to be combined into a single functional component with inlets and outlets. Only two design elements are required, cavities and through-holes. Besides the creation of the structures, the other vital engineering technique is that of the achievement of a hermetically sealed bond between the individual layers, impermeable to chemicals. The design of a simple microreactor with a Y-junction to enable two fluid substances to be brought together will be taken for the purposes of illustration. In addition, it is possible to include two further functional layers to control the temperature in the microreactor, or alternatively, to operate it directly within a thermostat. The model microreactor is defined by its individual layers, where each bear a structure in a two-dimensional pattern, with a given height as the third dimension, Figure 3.1.
Figure 3.1 Setup of a simple microreactor with a Y-junction.
3.1.1 Glass As Material
Glass is much used as a material for technological purposes. One obvious type of application is in optics; however, it has also had a huge influence on the progress in chemistry, pharmacology, electrical engineering, and electronics. Its traditional use in the technical fields of laboratory experiment, electron tubes, and lamps has led to a huge variety of special types of glass for technical purposes because of the different chemical or physical properties demanded. The newer applications such as microsystems engineering have continued to extend the shaping processes employed. Glass is obviously appreciated for its chemical resistance, but its transparency is also a great advantage, allowing observation and even analysis from outside.
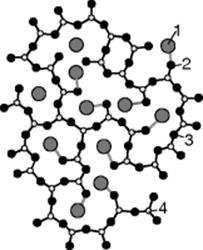
As a substance, glass is a noncrystalline solid with similarities to a liquid. A simplified representation would be to say it is made up of an irregular network of particles (usually SiO2), into the gaps between which the other components (the network converters, such as Na2O, K2O) are woven (Figure 3.2).
Figure 3.2 1: Network converters (Na2O), 2: oxygen, making no bridges, 3: oxygen, making bridges, 4: SiO2, making bridges).
The mechanical, chemical, and thermal properties decide the structuring possibilities. The high structural resistance that glass and glass ceramics possess (σB > 104 N/mm2) is not of practical significance as the resistance to breakage of actual articles made of glass always depends on manufacturing defects in their surface. Instances of slight surface damage in the form of fine notches and cracks cause an article to break, because excessive increases in tension develop at the ends of the cracks when they are subjected to mechanical loads. In ductile materials such as metals, a plastic type of flow will dissipate this excess tension. Glass and glass ceramics, on the other hand, behave as if they were brittle. At the temperatures and for the time spent under pressure in “normal use,” these materials do not manifest any plastic flow that would enable the peaks of stress at the tips of cracks and notches to be dissipated.
Owing to its chemical resistance, glass is generally excellent for water, saline solutions, acids, organic substances, and even alkalis, so that in all these cases, it is superior to most metals and plastics. The only chemicals that have a noticeably adverse effect on it are hydrofluoric acid, strongly alkaline solutions, and concentrated phosphoric acid, particularly at high temperatures.
As its heat conductivity is low (typically 0.9–1.2 W/(m K) at 90 °C), changes of temperature within the glass will cause relatively steep temperature gradients. On heating, expansion tends to generate high mechanical tension. Significant is the low capacity for heat conduction and the lack of ductility. This is the cause for the low thermal shock resistance. This disadvantage can be offset by a low coefficient of thermal expansion. Of the various types of glass, fused silica is the one with the lowest specific thermal expansion and the highest resistance to changes in temperature. Glass would only be a second choice in situations where highly efficient heat transfer is required, as its thermal conductivity is low.
Despite its high chemical resistance and structural stability, its tendency to fracture under tension and the often inadequate resistance to temperature changes make glass more difficult to be structured using the classic structure-imposing methods. Wet and dry chemical etching is only possible within limits, but it produces good geometric resolution while the depth of the structure remains shallow. Inadequate resistance to temperature changes also complicates laser ablation, which can be used successfully only in the case of fused silica, the type with the lowest coefficient of thermal linear expansion. Of the machining methods, which rely on a shaving process, with few exceptions, it is possible to use only those in which the cutter operates with indefinite geometry, that is, lapping, ultrasound lapping, grinding, and sandblasting on a miniature scale.
One exception is the special type of photostructurable glass. For the construction of microreactors, the borosilicate types of glass are the most important, but also fused silica.
· Fused silica is a single-component type, which consists in of SiO2. Its technical importance lies mainly in its low coefficient of thermal expansion, its excellent high-temperature resistance (up to 1000 °C), its very high resistance to changes of temperature, and its extremely good transparency to ultraviolet light.
· Borosilicate glass will contain a higher proportion of SiO2 than most other varieties of silicate glass, together with varying amounts of B2O3, constituting up to 13% of the mass. This type is characterized by high resistance to the influence of chemicals and to differences in temperature. It thus finds application mainly in the chemical and pharmaceutical industry and as domestic ovenproof glass. The chemical composition can vary widely. What will decide the actual properties is mainly the manner in which the boron compounds suited to the glass melt are combined with other metallic oxides. Borosilicate 33 is used particularly often; the 33 stands for the thermal coefficient of expansion, α = 3.3 × 10−6 K−1, and the trade names often met are Borofloat 33, Duran, and Pyrex.
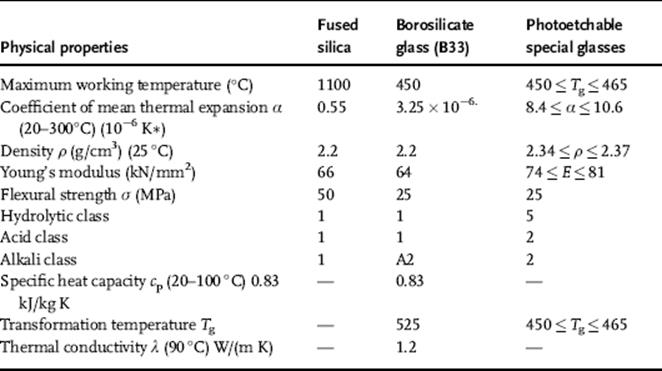
· Photoetchable special glasses belong to the doped lithium-aluminosilicate group. It is characteristic for this group to crystallize on the areas exposed to light if, after masking, they are subjected to UV-irradiation and then heated. The crystallized parts are more easily dissolved in hydrofluoric acid, so that a geometric microstructure can be created on the glass by this means. Some of the types that have made an impact in this field are FOTURAN (made by Schott, Germany) and PEG3 (made by Hoya, Japan). The Department of Inorganic Non-Metallic Materials at the Technische Universität Ilmenau is using a type of photostructurable glass (FS21) it has developed for its current research. Table 3.1 shows the most important properties of this glass [1].
Table 3.1 Physical properties.
It is available in wafer form but also with a rectangular shape. The structuring methods most often used are described below.
3.1.2 Silicon As Material
Silicon as a material is very common in microsystems engineering as it is suitable not only for microswitches but also successfully applied in the creation of structures for mechanical purposes and fluids. The reasons lie not only in its useful mechanical properties but also in its ready availability and its ease of structuring.
Silicon is used in its monocrystalline form. The single crystal is developed by a variety of processes, as a cylindrical ingot, or boule. This cylindrical shape is ground to a nominal diameter and then sliced. The resultant wafers are polished. Silicon crystallizes with a diamond structure. The wafers may have the (100), (110), or (111) orientation; the (100) orientation is most commonly used. Figure 3.3 shows the layers in a cubic crystal system, described by their Miller indices, (6). The most important properties are given in Table 3.2.
Figure 3.3 Layers in the cubic crystal system.
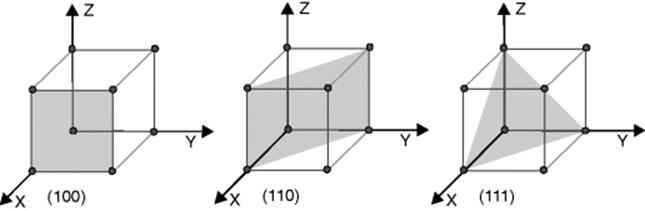
Table 3.2 Physical properties of silicon.
|
Physical properties of silicon |
Silicon |
|
Coefficient of mean thermal expansion α (20–300 °C) (10−6 K*) |
2.6 |
|
Density ρ (g/cm3) (25 °C |
2329 |
|
Flexural strength σ (MPa) |
6000 |
|
Thermal conductivity λ (90 °C) W/(m K) |
150 |
|
Young's modulus (GPa) |
130–188 |
|
Melting point (°C) |
1413 |