Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
5. Homogeneous Reactions
5.2. Base-Promoted Reaction
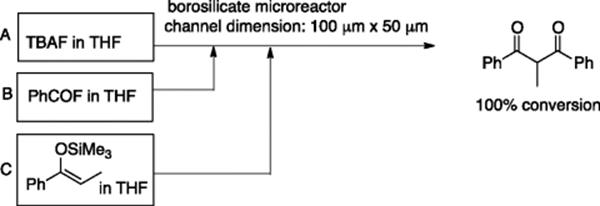
The combination of fluoride ions and enol silyl ethers provides a useful method for the generation of enolate anions [13]. Watts, Haswell, and coworkers applied a borosilicate glass microreactor, with channel dimensions of 100 μm × 50 μm, equipped with an EOF (electro-osmotic flow) pumping system, to the C-acylation of enolate anions, which leads to 1,3-diketones [14, 15]. A THF solution of tetrabutylammmonium fluoride (TBAF) was placed in reservoir A, a THF solution of benzoyl fluoride in reservoir B, and enol silyl ether of propiophenone was placed in reservoir C. The desired 1,3-diketone was formed in 100% conversion (Scheme 5.11). As for enol silyl ether of acetophenone, benzoyl cyanide was used to obtain the corresponding 1,3-diketone, since the use of benzoyl fluoride resulted in the selective formation of the corresponding vinyl acetate, the O-acylation product. A similar EOF-based microreactor was tested for Michael addition of β-diketones and diethyl malonate to ethyl propiolate and methyl vinyl ketone. Ethyldiisopropylamine in ethanol was used as the solvent, and a stopped-flow technique was employed to increase conversion efficiency [15].
Scheme 5.11
More recently, Löwe and coworkers reported a detailed study on the addition of secondary amines to ethyl acrylate and acrylonitrile using a continuous microreaction process based on an IMM micromixer and tabular reactor [16]. In the best case, space-time yields (g/ml h) for the microflow system were much higher than those for the batch system, by a factor of ~650.
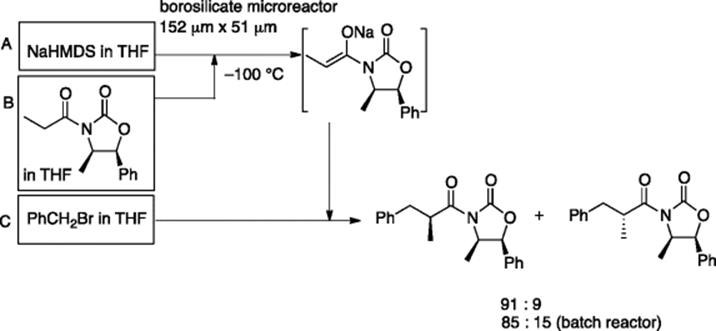
A borosilicate glass microreactor (152 μm (width), 51 μm (depth), and 2.3 cm (length)), which was connected to a T-shaped PEEK unit (MicroTee, Upchurch Scientific), was used for the formation of sodium enolate of N-propionyloxazolidinone and the subsequent diastereoselective alkylation with benzyl bromide at −100 °C. The observed diastereomeric ratio of 91: 9 was superior to that of 85: 15 observed in a batch reactor (Scheme 5.12) [15, 17].
Scheme 5.12
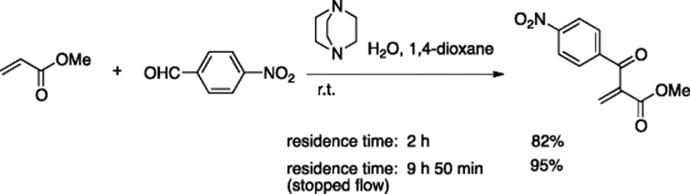
The DABCO-promoted Baylis–Hillman reaction was run continuously using the CYTOS College System with yields comparable to those of the batch reaction and with a significant reduction in reaction time (Scheme 5.13) [18]. Coupled with the stopped-flow technique, almost complete conversion was achieved.
Scheme 5.13
Kitazume and coworkers used microreactors with microchannels 100 μm wide and 40 μm deep for the synthesis of a series of organofluorine compounds [19, 20]. The silylation of 4,4,4-trifluorobutan-2-one and the Mukaiyama-type aldol reaction of the resulting enol silyl ether with acetals gave good yields of the desired products [20]. They also described nitro-aldol reactions of 2,2-difluoro-1-ethoxyethanol and Michael additions of nitroalkanes to ethyl 4,4,4-trifluorocrotonate and ethyl 4,4-difluorocrotonate [19, 20]. Reactions were carried out at room temperature, and the yields generally were comparable to those obtained in batch reactions. The following example demonstrates trifluoromethylation of benzaldehyde using trifluoromethyl(trimethyl)silane in the presence of TBAF (Scheme 5.14) [19].
Scheme 5.14
