Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
1. Properties and Use of Microreactors
1.2. Physical Characteristics of Microreactors
1.2.1 Geometries
1. Size: Microreactor systems incorporate structures for the directed transport or containment of gases or fluids that have a dimensional property in at least one direction usually measured in micrometers, sometimes up to 1 mm. These structures may comprise microscale ducts (e.g., channels and slots) and pores, larger features (e.g., parallel plates) that cause fluid to flow in thin films, and others that cause fluid to flow in microscale discontinuous multiphase flow (e.g., bubbles and emulsions). More specific details of these types of structure are explained in Chapters 9 and 10. In addition, small containment structures such as microwells have been fabricated in an analogous format to traditional microtiter plates, rendering potential compatibility with existing robotic handling systems as used in many high-throughput screening laboratories. Extending the notion of a microreactor, an increasing number of studies are demonstrating how separated droplets may act as nanoscale-based reactors [39]. For instance, the use of solvent droplets resulting from controlled segmented flow has been proposed as individual nanoliter reactors for organic synthesis [40–42]. Similarly, reverse micellar structures have been shown to provide reactors for the controlled synthesis of nanometer-scale particulates [43, 44]. Also, giant phospholipid liposomes (~10 μm diameter) have been utilized as miniature containers of reagents and can be manipulated by various external mechanisms, such as optical, electrical, and mechanical displacement and fusion [45]. Liposome-based microreactors, manipulated in this manner, hold the potential to enable highly controlled and multiplexed microreactions in a very small scale [46].
2. Architecture: Geometries employed in microreactor design and fabrication may range from simple tubular structures, where perhaps two reagents are introduced to form a product, to more sophisticated multicomponent circuits, where several functionalities may be performed, including reagent injection(s), mixing, incubation, quench addition, solvent exchange, crystallization, thermal management, extraction, encapsulation, or phase separation.
3. Multiplicity: Microreactors may comprise single-element structures from which small quantities of reaction products may be obtained, or, massively parallel structures where output on an industrial scale can be realized. Examples of numbering-up of microreactors are shown in Figure 1.7. In Figure 1.7a, 10 glass microreactors are placed on top of each other to form one single, multileveled device [47]. The microchannels were produced by photolithography and wet etching, and each glass reactor was thermally bonded together. The microreactors were used for the production of amides, and using this numbering-up technique, the authors found a 10 times higher throughput yielding product on the scale of grams per hour [47]. Figure 1.7b shows another example of paralleled microreactors, named the Cambridge Disc Microreactor systems [48]. Ten capillaries of 200 μm in diameter and 30 m in length were lined up and embedded in a polymer film and then wound into a disc-shaped device. This system can be used to perform organic synthesis reactions at temperatures up to 150 °C [48].
Figure 1.7 Examples of multiple microreactors used in parallel for higher throughput and yield of products [47, 48]. Source: Figures reprinted with permission, copyright (2010), American Chemical Society.

The principle of numbering-up has been used on an industrial scale for nitration reactions performed under current good manufacturing practice (cGMP) [49]. Historically, in 2001, CPC built and commissioned one of the first microreactor-based micromanufacturing plants, which incorporated many parallel microreactors. This was for the manufacture of diazo pigments for the company Clariant. The three-step manufacturing process, which involved (i) diazotation, (ii) coupling, and (iii) pigmenting (conditioning), was found to improve the product quality through improved particle size distribution and dye properties of the diazo pigments. The so-called CYTOS Pilot System used multiples of individual microreactors, so that products developed as small quantities on a laboratory scale could be produced in bulk at a manufacturing level without changing the essential chemical processing conditions. The scale of such a system is shown in Figure 1.8.
Figure 1.8 Parallel microreactor system (the so-called CYTOS Pilot System) designed and commissioned by CPC for Clariant in 2001, for the manufacturing of diazo pigments. Source: Reprinted from Ref. [50], Copyright (2007), with permission from Elsevier.

The engineering of numbering-up solutions for processes involving reactions with heat/mass transfer usually requires a distribution system from a common reactant source through many reaction microchannels to a common product outlet such that the same residence time is experienced in all the reaction microchannels. From an analytical comparison of bifurcating and consecutive source/outlet manifold structures, as resistance networks, design guidelines have been derived, which consider manufacturing variations in microchannel geometry, microchannel aspect ratio, and microchannel blockages incurred during function [51, 52]. From this, it has been shown that a distribution system of bifurcating ducts always produces flow equipartition as long as the length of the straight channel after each channel bend is sufficient for a symmetrical velocity profile to develop. Nevertheless, it is clearly important to be able to detect blockages within channels, but placing sensors in every channel is not economically feasible. However, one scheme has shown that by careful consideration of circuit design, blockages in any one of a number of parallelized microreactors can be detected with just two in-line flow sensors [53].
A long-standing issue in the development of process chemistries is that a reaction scheme developed in a small bench top flask may not scale-up with the same output parameters when transferred to an industrial production reactor. Instead, this problem is potentially circumvented by arithmetically numbering-up, in parallel operation, the multiplicity of the same microreactors to achieve the target output [1]. However, this engineering challenge is not trivial, since many parallel reactors may be required to achieve significant volume outputs. Pioneering examples of those industrial processes, which have been successfully achieved using microreactor technology, are described in Chapter 5. Those, which have shown commercial success, appear to represent mostly high-value, relatively low-volume products, products that are particularly dangerous to manufacture, entirely new class of products, and/or those that have a short shelf life.
1.2.2 Constructional Materials and Their Properties
Microfluidic devices, which may be suitable for chemical synthesis according to the processing conditions, have been fabricated from a range of materials including glass [54], elastomers [55], silicon [56], quartz, flouropolymers [57], metals [58], and ceramics [59] employing the techniques of laser ablation [60], wet chemical etching [61], abrasive micromachining [61], deep reactive ion etching [62], molding [63], embossing [64, 65], casting [66], and milling [67].
Advanced microreactors for manufacturing-level chemical production place demanding requirements on their integrated functionality and durability. For instance, thermal tolerance to processing conditions, temporal stability of surface energy, surface chemistry, and activity of incorporated catalysts and compatibility with sterilization protocol are important considerations when choosing a constructional material. Materials are also required to [68]
· be chemically inert,
· have appropriate thermal and electrical properties,
· be compatible with solvents and acids used,
· have some degree of light transparency if on-chip analysis is required,
· be compatible with fabrication protocol employed,
· be usable for extended processing durations.
These are further complicated by application- and process-specific requirements. For example, the excitation and control of reactions may require temporal and spatial modulation of applied energies such as UV, IR, and microwave radiation. All these considerations place exacting specifications on the constructional materials in order that the required geometries may be fabricated in a manner that is cost-effectively compatible with envisaged manufacturing-level scenarios. Where massively parallel microreactor systems are required for volume outputs, constructional materials must be appropriate to the economies and micromanufacturing processes of mass-fabricated parts. Equally, levels of specific functional integration must be equated with the overall system-level integration strategy and range from monolithic to hybrid solutions. Most preferably, this is more of a long-term goal, reconfigurable or addressable component functions will allow for the creation of application-specific microreactor ensembles from a “programmable” platform technology.
Table 1.1 gives a brief overview of the different materials, advantages and disadvantages, fabrication techniques, and applications in microreactor technology. Glass and silicon have been used extensively in earlier microreactors and are slowly being supplemented by inexpensive and easy-to-fabricate polymers such as polydimethylsiloxane (PDMS), at least in academic research laboratories [69]. Many copies of a PDMS microfluidic circuit can be molded from a master made from, for example, silicon [70]. However, there are limitations in its use, and for microreactors, it is generally the swelling of the PDMS in a solvent, which first limits its application [71]. Glass is still the material of choice for many synthetic applications due to its characteristics described in Table 1.1 [68, 72]. However, due to its low thermal conductivity, glass is not quite as suitable for high-temperature and high-pressure reactions. Stainless steel, silicon, and ceramics are the alternative materials that can be used for these specific reactions [68]. More details on particular important aspects are described in Chapters 2–4 and in the given references.
Table 1.1 Overview of materials and microfabrication techniques used for microreactor construction [68, 69, 72–75]


While basic microreactors and arrays may be fabricated from glass, polymers, metals, or ceramics, advanced microreactors with multifunctional and reconfigurable capability will require construction from a diverse and integrated materials set. As example, focused microwave excitation delivered at multiple resonator nodes within a fluidic microreactor array will require constructional materials and associated machining processes suitable for both reaction chemistry and the spatial distribution of microwave energy. For this, a set of glass, polymers, and metals are required each of which might be separately microstructured using one or more techniques of subtractive machining (etching, ablation), embossing, molding, and casting. Industrial-scale processes in microreactors are often conducted under medium to high pressures and with the use and production of highly reactive chemicals. This may require the use of pressure-, solvent-, and temperature-tolerant stainless steel, ceramic, or glass with associated accessories, such as gaskets and interconnects, sometimes fabricated from polytetrafluoroethylene and polyetheretherketone (PEEK). Although fluorous polymers might be an optimal choice for many applications including corrosive or other hazardous chemicals, their micromanufacturing compatibility must be taken into account. For instance, PTFE does not lend itself to the mass-fabrication technique of embossing but can be microstructured using reactive ion etching as used frequently at a wafer level with silicon. In contrast, a thermoplastic variant, perflouroalkoxy, can be molded; it is highly solvent resistant, has FDA approval for many applications, and is sterilization compatible. In contrast to the requirements imposed by industrial application, experimental, laboratory chip-based devices for research purposes have also been fabricated from the same materials but may also include silicon, silicon-pyrex, and occasionally polymers such as poly(methyl methacrylate), polycarbonate, cyclic olefin copolymer, and polydimethylsiloxane.
Many chemical reactions performed in microreactors are conducted at room temperature, but in others that require heating and/or cooling, thermal transfer to the microdevice is an important issue and imparts on the selection of constructional materials [76]. In this respect, cooling or heating units have been combined with microdevices to allow constant reaction temperatures or controlled temperature zones [77]. In the synthesis of biologically active fluorescent quantum dots, three separate microreactor chips were used, at different temperatures for (i) the control of the size and spectral properties of cadmium selenide and cadmium telluride nanoparticles at 300 °C, (ii) their zinc sulfide capping at 110–120 °C, and (iii) ligand replacement at 60 °C. These could, of course, be potentially integrated into one larger chip with zoned temperature control [78].
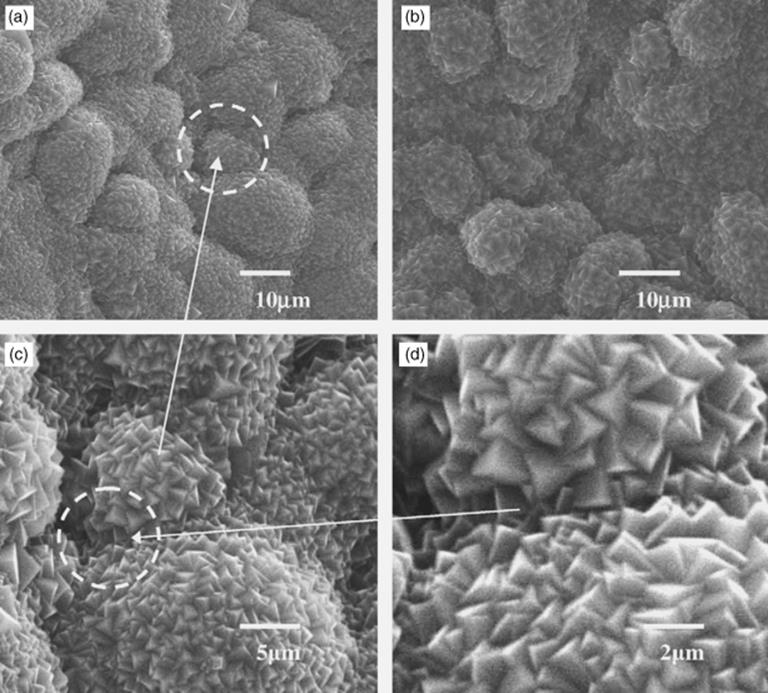
As well as the basic materials from which a microreactor is fabricated, there may be additional materials that are included as coating or packing. For instance, in a glass-polymer composite continuous-flow microreactor, palladium particles have been loaded by ion exchange and reduced. This was used in a Heck reactions and demonstrated to be re-usable for >20 times post wash treatment [79]. Also, coating of the capillary channel of a microreactor with elemental palladium allowed palladium-catalyzed coupling reactions to be performed very efficiently, and the metal coating also serves as recipient for microwave energy allowing a fast heating of the reaction solution [80]. Another popular coating is TiO2 that is frequently used as a photocatalyst for the degradation of organic pollutants. This has been coated onto prefabricated ZnO nanorods on the internal walls of a glass microreactor and has been shown to significantly increase the surface area for photocatalytic oxidation [81]. As a simpler one-step method of increasing the catalytic surface area, a foam-like porous ceramic containing a catalyst as nanoparticle was formed in a microreactor by direct sol-gelation, thus avoiding any separate coating or impregnation step [82]. The result demonstrated a reasonable pressure drop due to its porosity, high thermal and catalytic stability, and excellent catalytic behavior in forming hydrogen and carbon monoxide-rich syngas from butane. Additionally, zeolite materials can function as a valuable adsorbent and catalyst in microreactors and their precision growth can be pre-seeded from nano-zeolites grafted on to silanised microreactor surfaces such as metal (Figure 1.9 [83]).
Figure 1.9 Scanning electron microscope pictures of an example surface modification, in this case, a NaA zeolite film grown on seeded porous stainless steel, multichannel plate using chloropropyl trimethoxysilane (CP-TMS) linkers (a), (c), (d), and aminopropyl trimethoxysilane (AP-TMS) linkers (b) [83]. Source: Reprinted from Yang, G. et al. (2007) A novel method for the assembly of nano-zeolite crystals on porous stainless steel microchannel and then zeolite film growth. J. Phys. Chem. Solids, 68(1), 26–31, with permission from Elsevier.
A porous organic polymer monolith may be formed within a microreactor to act as a support for catalysts, such as palladium. The size, size distribution, and surface area of the pores may be controlled by a porogen, while the chemical properties are controlled by the monomer used. Such supports can be formed and even patterned by the use of ultraviolet light, most cost-effectively using ultraviolet light-emitting-diode arrays [84, 85]. Carbon nanofibers can also be deposited within microreactors by homogeneous deposition precipitation and pulsed laser deposition to provide a larger surface area support layer upon which catalysts such as ruthenium catalytic nanoparticles can be attached [86].