Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
5. Homogeneous Reactions
5.3. Radical Reactions
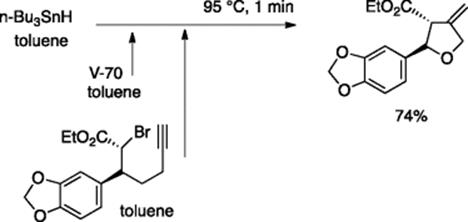
Tributyltin hydride is among the most popular reagents for radical chain reactions. The typical reaction time used for tributyltin hydride-mediated radical reactions in a batch flask reaction ranges from 10 min to hours in the literature. Ryu and coworkers examined tin hydride-mediated radical reactions of organo halides using flow microreactor and found that a few minutes were sufficient for the reaction [21]. This was beneficial when radical initiators that decompose more quickly than AIBN, such as V-65: 2,2′-azobis(2.4-dimethylvaleronitrile) and V-70: 2,2′-azobis(4-methoxy-2.4-dimethylvaleronitrile), are employed; the reaction is complete within 1 min to give the product in high yields. For example, tin hydride-mediated flow radical cyclization for the synthesis of a tetrahydrofuran derivative, which is a key intermediate for the synthesis of furofuran lignans, was carried out using a microflow system consisting of serially connected two micromixer (MiChS α) and a stainless-steel-made tube reactor (Scheme 5.15). Radical cyclization of alkynyl-substituted bromo ester using tributyltin hydride took place with 1 min residence time to give the desired methylene tetrahydrofuran in good yield. Continuous operation for 185 min gave 7.6 g of the tetrahydrofuran derivative.
Scheme 5.15
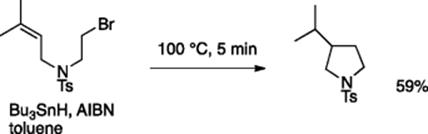
As already stated, monolithic and flexible polyimide (PI) film microreactors, which can be easily fabricated by photo-ablating a PI film, are durable under highly acidic conditions and also at reasonably high temperatures (up to 100 °C) [11]. Tributyltin hydride-mediated radical reaction proceeded smoothly with the use of a PI film reactor. For example, the radical cyclization product was obtained in 59% yield using the standard tin hydride/AIBN conditions (Scheme 5.16).
Scheme 5.16
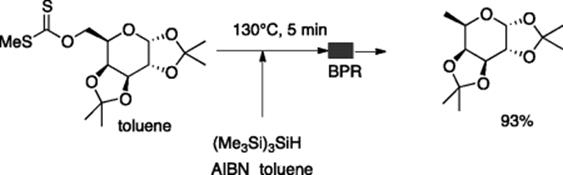
Tris(trimethylsilyl)silane (TTMSS) is a greener substitute for tributyltin hydride because of its low toxicity. Seeberger and coworkers reported the TTMSS-mediated radical reduction of alkyl halides and alcohol-based thiocarbonyl derivatives (Barton–McCombie deoxygenations) using a Syrris glass microreactor with a 1.0 mL heated retention unit connected with a back pressure regulator (BPR), which enabled the reaction to occur at a temperature higher than the boiling point of a solvent [22]. The deoxygenation of a xanthate derived from d-galactose gave 6-deoxy-d-galactose in high yield (Scheme 5.17).
Scheme 5.17
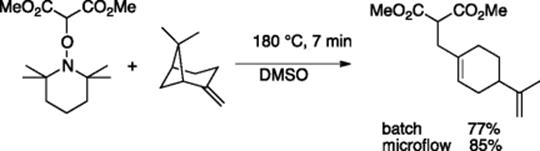
Alkoxyamines are known to undergo thermal radical addition reaction to alkenes. High thermal efficiency of a microflow system over a batch system was shown in carboaminoxylation of alkenes using TEMPO-malonate [23]. In the batch system, reaction of α-pinene with TEMPO-malonate for 10 min gave the addition/elimination product in 77% yield, whereas the corresponding microflow reaction gave the product in 85% yield (Scheme 5.18). It is noteworthy that microflow reactions gave negligible formation of the byproduct, which would be obtained through thermal decomposition of the primarily formed addition product. As the reaction mixture is immediately cooled as it exits from the microreactor, thermal decomposition of the target product is minimized.
Scheme 5.18