Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
5. Homogeneous Reactions
5.5. Metal-Catalyzed Reactions
The excellent diversity and enormous potential of metal-catalysis to construct chemical bonds prompted many researchers to employ microreactors for such reactions. The Sonogashira coupling reaction of aryl or vinyl halides with terminal alkynes is typically carried out in the presence of a Pd catalyst and a Cu co-catalyst. In 2002, Ryu and coworkers reported that the Sonogashira coupling reaction, using ionic liquid [bmim]PF6 (1-butyl-3-methylimidazolium hexafluorophosphate), proceeded smoothly without a Cu co-catalyst. The Cu-free Sonogashira coupling reaction using an IMMs interdigitated microreactor (40 μm channel-width) was examined [45]. When a solution of [bmim]PF6containing a Pd N-heterocyclic carbene complex and a mixture of iodobenzene, phenylacetylene, and dibutylamine, was mixed using a IMM micromixer at 110 °C with 10 min residence time, the coupling product was obtained in 93% yield (Scheme 5.33). After successive biphasic treatment with hexane and water, the Pd-containing ionic liquid solution could be reused leading to 83% yield of diphenylacetylene in the second reaction. A microflow system, requiring lower CO pressures than a batch system, was used for the carbonylative Sonogashira coupling reaction, yielding acetylenic ketones [46].
Scheme 5.33
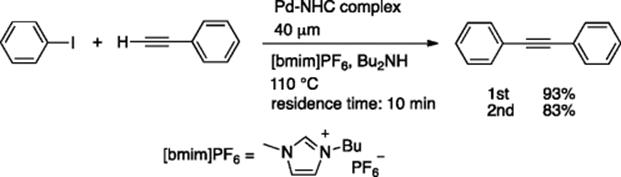
A fully automated microreactor-based system was developed for the Pd-catalyzed Mizoroki–Heck reaction, in which integration of all the basic steps, that is, reaction, separation of the product from the ionic liquid phase, recycling of the ionic liquid containing the Pd catalyst, could be realized in a completely continuous fashion [47]. A “bench-top” continuous production system was constructed using a continuous microflow reactor, a CPC CYTOS Lab System, and an originally developed workup protocol, based on a dual microextraction system using T-shaped micromixers (300 μm internal diameter [i.d.]). Low viscosity ionic liquid, [bmim]NTf2, ensured a smooth flow of the catalyst solution. After extraction using double T-shaped mixers, the ionic liquid containing the Pd-catalyst was continuously recycled using a pump. After running the system for 11.5 h, during which time 144.8 g (0.71 mol) of iodobenzene was consumed, 115.3 g of trans-butyl cinnamate was obtained (80% yield, 10 g/h) (Scheme 5.34). The ionic liquid with the Pd-catalyst was recycled about five times during this catalytic reaction.
Scheme 5.34
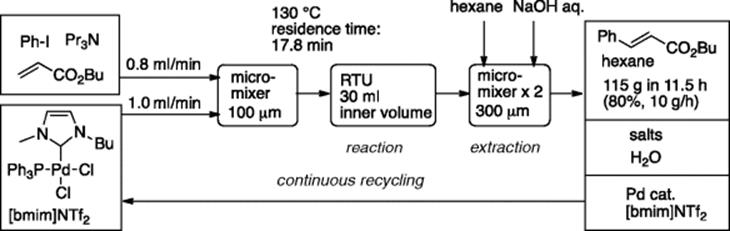
A matrix metalloproteinase inhibitor can be synthesized by Pd/Cu-catalyzed Sonogashira coupling reaction of bromothiophene having a tryptophan moiety with tolylacetylene in DMF [48]. In this “bench-top” continuous production-catalyst recycling system, Cu-free Sonogashira coupling reaction was conducted in a low viscosity ionic liquid [emim]NTf2 that acted as the reaction medium as well as catalyst support [49]. When the system was operated for 5.5 h, 103 g of the key coupling product was obtained (Scheme 5.35). The obtained coupling product could be transformed to the matrix metalloproteinase inhibitor in two steps.
Scheme 5.35
Patel, Mainolfi, and coworkers reported the Sonogashira coupling reaction using copper tube flow reactor (CTFR) without Pd catalyst [50]. Using CTFR having 1 mm i.d. (20 ml inner volume) with Vapourtec R4/R2 plus flow chemistry system, the reaction of o-trifluoromethyliodobenzene with phenylacetylene in the presence of tetrabutylammonium acetate (TBAA) in DMF at 170 °C was complete with 30 min residence time to give the coupling product in 92% yield (Scheme 5.36). In this reaction, CTFR acts as a source of copper catalyst. Ullmann coupling of iodobenezenes with amines and decarboxylation of benzoic acids were also carried out using the CTFR system.
Scheme 5.36

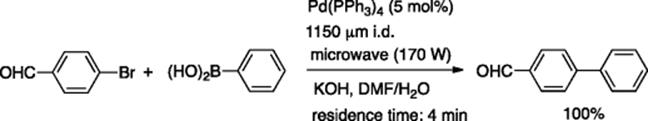
Several reports appeared on the microflow version of another important Pd-catalyzed reaction: Suzuki–Miyaura coupling. Lee, Valiyaveettil, and coworkers reported that a glass capillary microreactor (400 μm i.d.) was available for the Suzuki–Miyaura coupling reaction catalyzed by Pd nanoparticles [51]. Organ and Comer reported the microwave-assisted Suzuki–Miyaura coupling reaction in a microflow system [52]. The continuous flow design consisted of a stainless steel holding/mixing chamber with three inlet ports connected to a simple glass capillary tube (1150 μm i.d.) located in the irradiation chamber. The microwave assisted flow reaction of p-bromobenzaldehyde with phenylboronic acid using Pd(PPh3)4 as the catalyst and KOH as the base gave quantitative yields of the coupling product with about 4 min residence time (Scheme 5.37). When the reaction was carried out using Pd(OAc)2, the Pd-catalyst decomposed and coated the capillary wall with a thin metal film, which also catalyzed the coupling reaction. The microwave-assisted microflow system also has been applied to ruthenium-catalyzed ring-closing metathesis.
Scheme 5.37
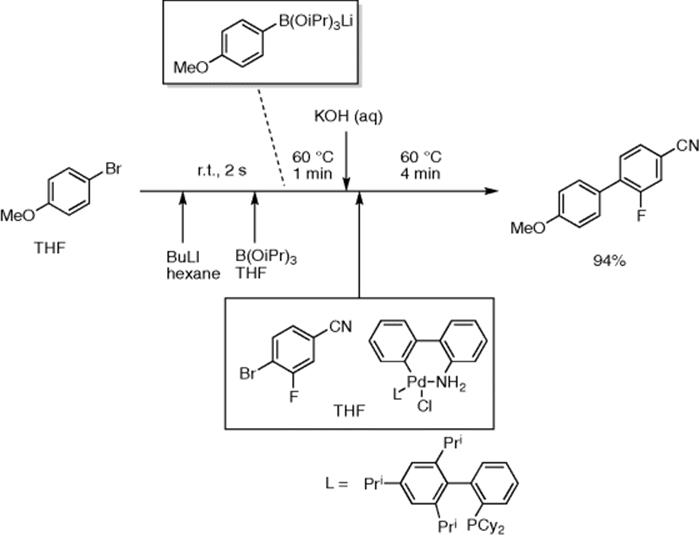
Buchwald and co-authors reported synthesis of biaryls by a lithiation/ borylation/Suzuki–Miyaura cross coupling sequence in continuous flow [53]. Aryl bromides could be lithiated at room temperature with 2 s–10 min residence time by mixing with n-BuLi, which then reacted with B(OiPr)3 at room temperature with 1 min residence time to afford aryl borates, ArB(OiPr)3Li. The reactor was sonicated to avoid blocking by precipitated ArB(OiPr)3Li. The reaction mixture was then mixed with Pd catalyst/THF solution and reacted at 60 °C (4–10 min residence time) to give biaryls in good yields. For example, 2-fluoro-4′-methoxy-[1,1′-biphenyl]-4-carbonitrile was obtained in 94% yield (4 min residence time) (Scheme 5.38).
Scheme 5.38
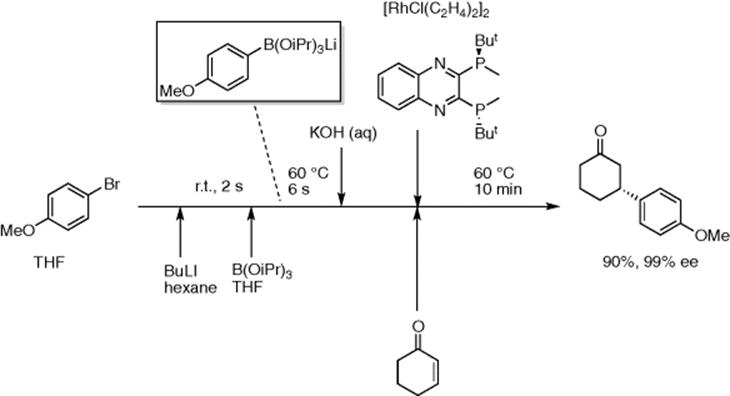
ArB(OiPr)3Li, generated from aryl bromides in a similar way, can be used for Rh-catalyzed asymmetric 1,4-addition to enones (Scheme 5.39) [54]. High yields of the products were obtained within 10–20 min residence time with high enantioselectivity.
Scheme 5.39
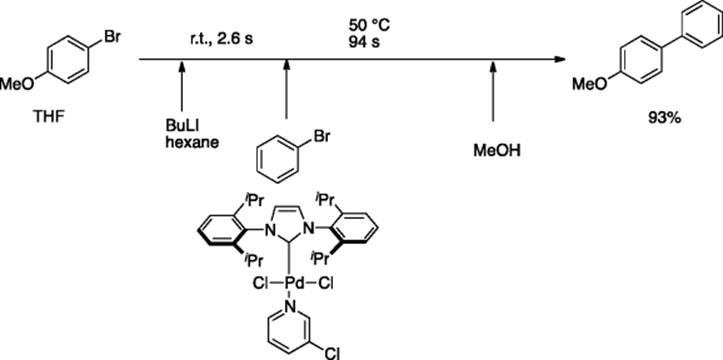
Yoshida and coworkers reported Pd-catalyzed Murahashi coupling reaction of aryl halides (Ar1Br) with aryllithiums, generated by lithiation of other aryl bromides (Ar2Br) with n-BuLi in a continuous flow system, where Pd complex, PEPPSI-SIPr ([1,3-bis(2,6-diisopropylphenyl)imidazolidene(3-chloropyridyl)palladium(II) dichloride]), was found to be a good catalyst [55]. The aryllithium generated by the reaction of bromoanisole with BuLi in the first reactor (500 μm i.d., 200 cm length) was mixed with a THF solution of phenylbromide (0.52 M) and PEPPSI-SIPr in a micromixer (250 μm i.d.) and then heated in a second reactor (1000 μm i.d., 2400 cm length). After being quenched with MeOH, the coupling product was obtained in 93% yield (Scheme 5.40).
Scheme 5.40
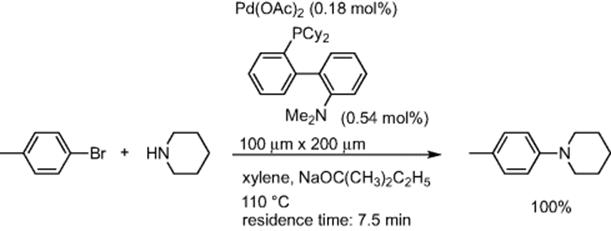
Palladium-catalyzed amination of aromatic halides, developed independently by Buchwald and Hartwig, is a useful tool for the construction of CߝN bonds. Caravieihes and coworkers reported using a microreactor for aromatic amination [56]. Using the CPC CYTOS Lab System, reaction of p-bromotoluene with piperidine gave the desired coupling product in quantitative yields with a 7.5 min residence time (Scheme 5.41).
Scheme 5.41
Suresh, Lee, and coworkers demonstrated oxidation of cyclohexene catalyzed by Mn- or Cu-complexes, using H2O2 in the aqueous phase, in a microreactor (width = 200 μm, depth = 50 μm) [57]. Water-soluble ionic liquid [bmim]BF4 was added (0.5% v/v) to improve the solubility of cyclohexene in the reaction buffer. Using a reduced Schiff base–Cu complex, 2-hydroxycyclohexanone was obtained as the major product with 25 min residence time, whereas the bulk scale reaction gave 2-cyclohexenol as the major reaction product.
Roberge, Fogg, and co-authors developed a continuous stirred-tank reactor (CSTR) system for ring closing metathesis [58]. While the ring closing metathesis of the diene in a continuous flow system proceeded smoothly, the yield and selectivity were adversely affected by co-entrapment of ethylene with the catalyst (up to 82%). On the other hand, in the CSTR system, since ethylene was efficiently swept out in the batch tank, a quantitative yield of the RCM product was obtained (Scheme 5.42).
Scheme 5.42
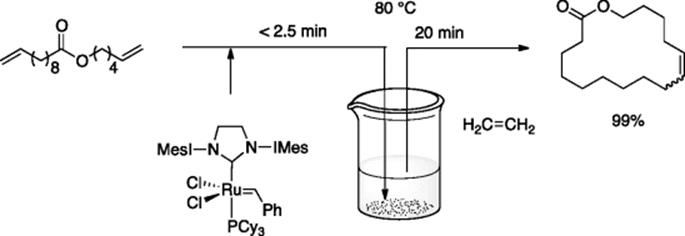
Kim, Grubbs, and coworkers reported microflow cross-methathesis of methyl oleate with ethylene [59]. The reaction in the microflow system gave the desired product in higher selectivity compared to the reaction in bulk batch system due to the high surface area-to-volume ratio suitable for fast mass transfer of the gaseous ethylene in to the solution phase.