Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
5. Homogeneous Reactions
5.7. Oxidation Reactions
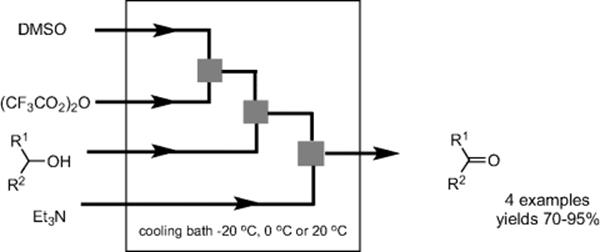
Oxidation of primary and secondary alcohols to aldehydes and ketones with DMSO, oxalyl chloride, and a base is known as Swern-oxidation. When using oxalyl chloride as the dehydration agent, the reaction must be kept colder than −60 °C to avoid side reactions, such as Pummerer rearrangement. In contrast, when trifluoroacetic anhydride is used instead of oxalyl chloride, the reaction can be warmed to −30 °C without side reactions. Microflow systems offer a smart approach for controlling the reaction temperature and allow precise control of the residence time. Yoshida and coworkers reported a microflow Swern-oxidation that worked well even at room temperature [67]. By sequential mixing of DMSO, alcohols and reagents, with the aid of a serially connected network of micromixers (IMM) and microtube reactors, carbonyl products were obtained in good to excellent yields, and formation of undesired side-products was minimized as well (Scheme 5.49). The reaction was efficient even at 20 °C, provided that the residence time was very short (2–5 s). Such a short reaction time ensures immediate transfer of the highly unstable intermediates for the subsequent reaction before their decomposition. In addition, the process withstood extended run times (run time = 3 h), allowing for potential scale-up.
Scheme. 5.49
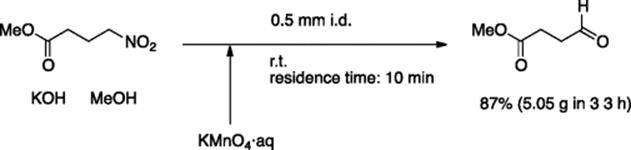
Nef oxidation was successfully carried out in a continuous flow system. Nitroalkanes were effectively transformed to aldehydes or carboxylic acids using KMnO4 as an oxidant, where the ultrasound pulses were applied to the flow system to avoid blocking by generated MnO2 [68]. The reaction of ethyl 4-nitrobutanoate (7.37 g) gave 5.05 g of the corresponding aldehyde in (87% yield) (Scheme 5.50).
Scheme. 5.50