Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
7. Heterogeneous Reactions
Kiyosei Takasu
Continuous-flow technology has been receiving increasing attention in recent decades [1–7]. A flow reactor allows good control over the reaction conditions, including heat transfer, reaction time, and mixing efficiency. The side reactions can be minimized in many cases owing to the fine reaction control. Thus, greater yields of products with higher purity can be expected in a flow reactor compared with a traditional batch reactor. Once the parameters for the flow reactions are optimized, high reproducibility of the reactions will also be achieved. In addition, a flow microreactor offers safety benefits. Since the reaction volumes in the reactor are very small, the quantity of hazardous and explosive reagents/catalysts can be reduced. Moreover, since multistep reactions can be arranged in a continuous sequence, the technology can be deeply connected with high-throughput synthesis, microscale combinatorial synthesis, and automated robotic synthesis.
Solid phase organic synthesis (SPOS) is a related technology to continuous-flow synthesis. SPOS is a method in which substrates are bound on a bead and synthesized step-by-step in a reactant solution. Because the desired product/intermediate is bound to a resin, it is easy to remove excess reactant or byproduct from the product. After the multi-step transformation is completed, the desired molecule is cleaved from the resin. This method was originally developed for the synthesis of peptides by Merrifield, who was a Nobel winner in 1984 [8]. Deoxyribonucleic acid (DNA) and other molecules can be synthesized based on the SPOS method. More recently, this method has also been used in combinatorial chemistry. However, the major problem with SPOS reactions is that often they are not easily monitored. It is clearly not possible to analyze the polymer-supported products without detaching them from the beads. An alternative to the SPOS method is a solid-phase-assisted solution-phase synthesis using polymer-supported reagents or catalysts. As the substrate and products are not immobilized, it is much easier to analyze the reaction course using the well-established methods available for soluble species. A variety of polymer-supported reagents/catalysts as well as solid-supported scavengers have been reported, and some of them are commercially available [9–12].
The chemical reactions are classified as homogeneous and heterogeneous, depending on whether chemical components, such as reactants, reagents, catalysts, and solvents, exist in the same phase. In the homogeneous reaction, all components are equally dissolved in a solvent or gas. From the theoretical standpoint, homogeneous reactions are the simpler of the two classes of reactions because the chemical changes that take place are solely dependent on the nature of the interactions of the reacting substances. In contrast, heterogeneous reactions, in which all the chemical components are not equally dissolved in one phase, occur at the surface of the two different phases through several physical and chemical interactions. Thus, the reactant diffuses to the reagent/catalyst surface and adsorbs on it. After the chemical transformation, the resultant product desorbs from the surface of the reagent/catalyst and diffuses away. The major advantage of the heterogeneous reactions over the homogeneous ones is the ease of separation of the reagent/catalyst from the reaction media at the end of the reaction. This eliminates or minimizes the need for additional work-up procedures that can often be expensive and can lead to generation of large volumes of waste. An additional advantage is that the recovered catalyst sometimes can be used again in the same reaction (recyclability). The recovered immobilized waste derived from the reagent can be regenerated by the appropriate chemical transformation and can then be used again. Due to these advantages, efforts have been made to develop heterogeneous reactions, especially catalysis, for sustainable chemistry. Many of the previously reported studies have focused on the performance of heterogeneous reactions using flasks and other conventional reaction equipment. However, the application of newer equipment, including flow reactors, to heterogeneous reactions could lead to a new horizon in the science of synthesis. This chapter summarizes the general flow synthesis tools for heterogeneous reactions as well as representative flow reactions [13, 14].
7.1. Arrangement of Reactors in Flow Synthesis
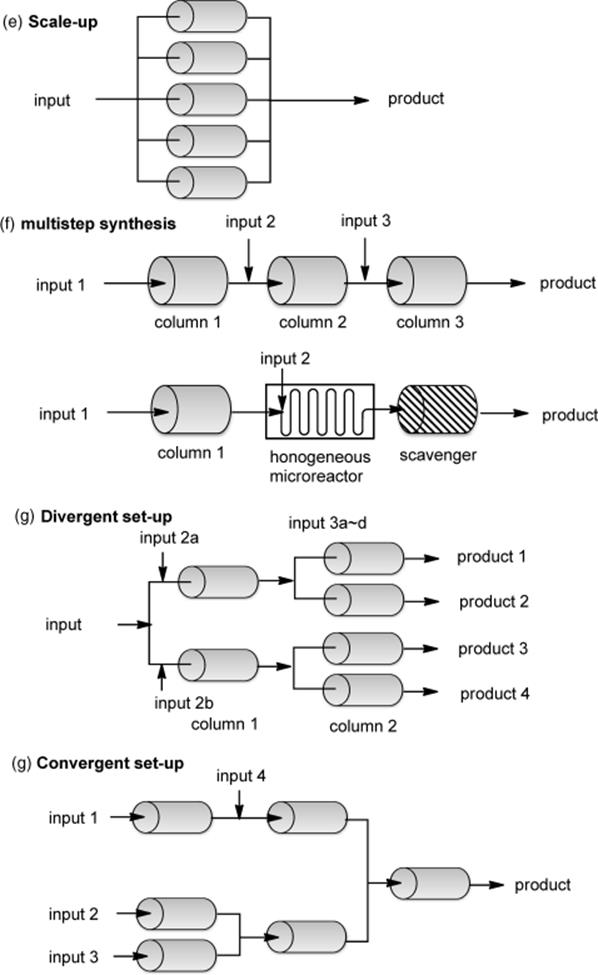
There are many possible combinations for heterogeneous flow reactions using solid-phase reagents/catalysts. Typical arrangements for heterogeneous reactions using a single reactor are illustrated in Scheme 7.1 [15]. (a) The most common and simplest system affords the reaction mixture continuously from the reaction column, in which a heterogeneous reagent/catalyst is immobilized. A single chemical transformation occurs in the reaction column. (b) In-line connection with an in situ analytical system such as UV-vis, Raman, IR, MS, HPLC, or an NMR spectrometer enables real-time monitoring of the reaction. This allows rapid analysis and optimization of the reaction conditions. (c) By incorporating a solid-supported scavenger for the removal of reaction waste such as excess reagent and by-products, a pure product can be obtained with no need for traditional purification procedures such as an aqueous workup, distillation, and column chromatography. (d) A system combined with LCMS and a fraction collector is also available for real-time monitoring of the reaction and in-line purification. (e) A flow reactor permits a recycling system of the flow stream. When the reaction rate with the polymer-supported reagent/catalyst is relatively slow, continuous recirculation of the reaction mixture through the column will be effective.
Scheme 7.1 Typical arrangements for a heterogeneous single-step flow reaction.
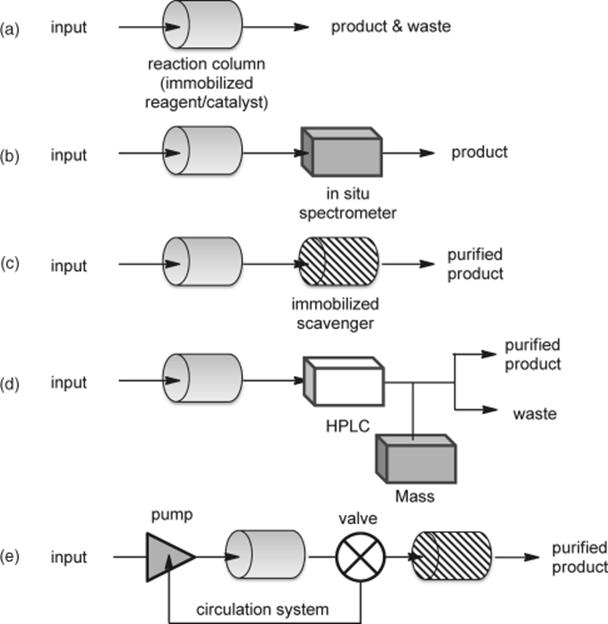
A flow system permits a combination of more than two flow reactors (Scheme 7.2). (e) Synthesis of a large quantity of the product is made possible simply by multiplying the number of flow reactors. The parallel arrangement of the immobilized reagent/catalyst is generally more efficient than the flow reaction using a single large column. The above-mentioned systems are for a single-step chemical transformation. (f) An in-series connection of more than two reactors (with, if necessary, appropriate scavenger columns) enables an arrangement of multistep reactions. Thus, space integration [16], in which a sequence of reactions is conducted in a flow system by adding components at different places, can afford more complex molecules from simple substrates. Of course, a heterogeneous flow system can be connected with a homogeneous or a bi-phasic flow reactor. (g and h) The flow reactor can be extended to an automated (or semi-automated) multistep synthesis. A divergent setup of the flow systems can be easily applied to a compound library synthesis. A convergent setup would be a significant technology for the industrial synthesis of pharmaceuticals.
Scheme 7.2 Advanced arrangements for different synthetic missions.