Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
7. Heterogeneous Reactions
7.2. Immobilization of the Reagent/Catalyst
Unfortunately, the widely used flow reactors (for example, microreactors, multi-cell flow reactors, and disk reactors) do not tolerate solid particulates and precipitates, which would clog the miniaturized flow devices. Therefore, immobilization of the reagents/catalysts by a solid support is of significant importance in this field. Hence, a broad range of solid supports have been employed to incorporate the reagent/catalyst into the reactors, including packed-beds, monoliths, and other systems that exploit the high surface-to-volume areas obtained in microchannel devices.
7.2.1 A Packed-Bed Reactor
A packed (fixed)-bed reactor is the simplest and most conventional method for immobilization of the catalyst/reagent [17]. A reaction solution passes over the retained reagent/catalyst particles in the packed-bed reactor. The chemical reaction takes place on the surface of the solid. The advantage of using a packed-bed reactor is the increased conversion per weight of catalyst compared with the corresponding batch reactions. These reactors show increased yield with lower amounts of by-products compared with the corresponding batch reactions. Packed-bed reactors are the most important type of reactor for the synthesis of large-scale basic chemicals and intermediates in industry.
A packed-bed reactor is a tubular vessel such as a column tube or a metallic cartridge in which the solid reagent/catalyst particles are randomly filled. The particles do not move with respect to a fixed reference frame. Micro- or nano-sized powder of a solid reagent/catalyst itself, such as metal or metal oxide, can be packed in the reactors. Polymer beads grafted with a reactive or catalytic moiety are also available. Silica gel (SiO2), alumina (Al2O3), perovskites (CaTiO3), activated carbon, and organic polymers are generally used as a solid phase. Recently, polyurea matrices and functionalized dendrimers have also been utilized in the flow reactions.
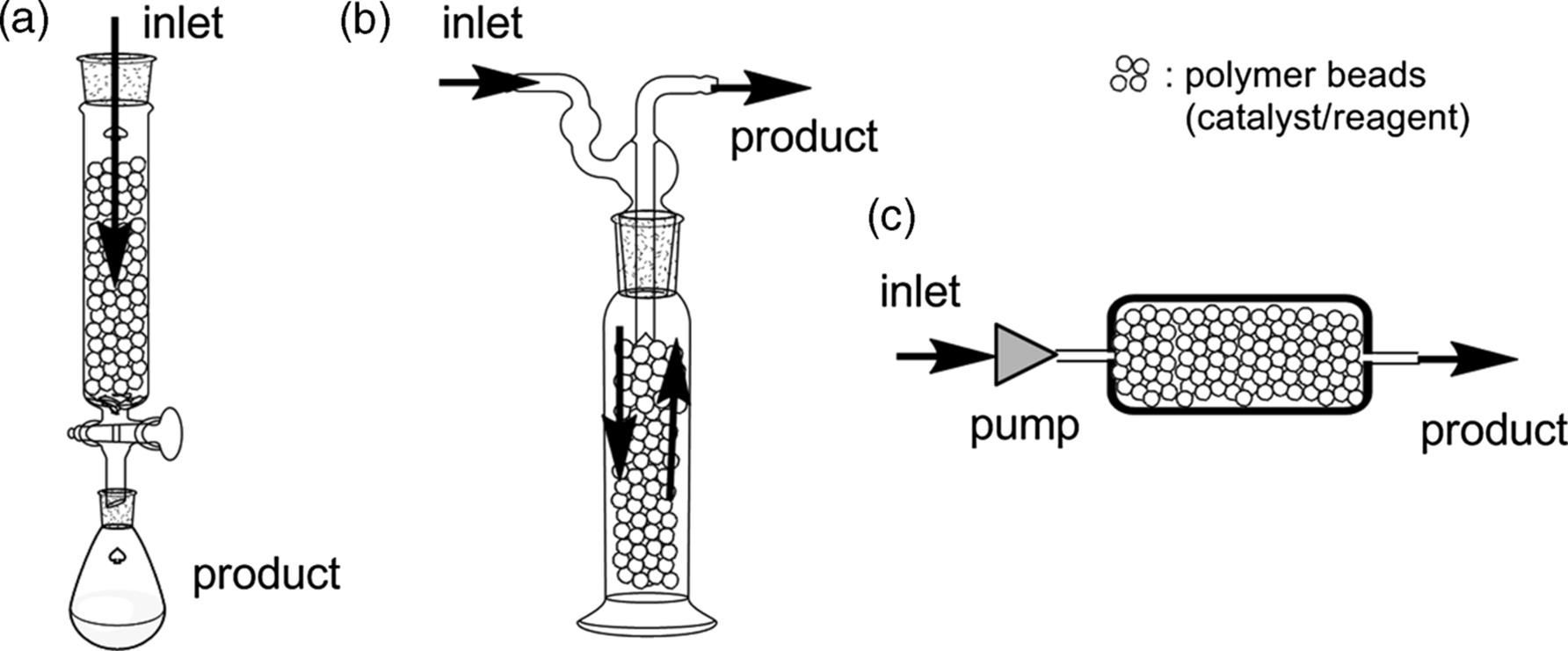
Several variations of packed-bed columns have been used in flow reactions, as shown in Figure 7.1 [18]. (a) In an early stage of heterogeneous flow chemistry, gravity-fed packed-bed columns, similar to those used for silica gel chromatography, were mainly used. Typically, the column would have a sintered glass frit to retain the beads and a tap to control the flow. (b) An alternative arrangement is the overflow-type reactor, which allows easy heating or cooling of the flow reaction vessel. (c) This type is essentially the same as the others, except that the reagent/catalyst particles are fixed in a pressure-resistant cartridge. Thus, the substrate phase can be continuously passed through the reactor by using a pump despite the high pressure.
Figure 7.1 Schematic representation of the different types of packed-bed reactors.
Although packed-bed reactors are convenient and rather cheap to prepare, there are some potential drawbacks. One is the swelling of the solid phase. If the swelling properties significantly change as the reaction proceeds, the bed volume will be changed and may lead to distract the packed-bed reactor. Another major problem is the homogeneity of a solid packing. Grain particles of 50–100 μm size are used and randomly fixed in the vessel for the common packed-bed reactors. When packing is irregular, these fixed beds show uncontrolled fluid dynamics that result in various disadvantages from a chemical reaction engineering standpoint. The formation of localized hot spots and clogging zones would cause broad residence time distribution, which could result in low process efficiency and selectivity due to poorly controlled fluid dynamics.
7.2.2 Monolith Reactors
To overcome the drawbacks of packed beds, numerous groups have focused on the use of macroporous monoliths in flow systems [19, 20]. A monolith is defined by IUPAC as a shaped, fabricated, intractable article with a homogeneous microstructure that does not exhibit any structural components distinguishable by optical microscopy. In general, a monolith comprises a honeycomb-like ceramic structure, covered by a thin (<50 μm, ideally 2–10 μm for a flow reactor) layer of a continuous uniform porous support material, which is prepared by precipitation polymerization of a functionalized monomer. Due to the high void volumes made by the extensive number of small pores or channels within a monolithic reactor, the pressure drop is commonly low during the passage of a fluid [21, 22]. The polymer particles can only swell and shrink in the pore volume available. The reactive moieties are packed not onto a solid support but on the inner walls of the microreactor itself. This generally solves the issue of localized hot spots and clogging zones. In addition, a large contact area of a reagent/catalyst with a substrate can be achieved [23].
Organic materials based on polystyrene- or polyacrylate-based copolymers and inorganic materials based on silica gel, zeolites, or carbon are available for the preparation of monoliths. Recently, metal-organic frameworks (MOFs) have received much attention as a new-generation monolith. Among them, organic monoliths are the most popular solid phase for heterogeneous reagents/catalysts in a flow mode. Three important strategies for the preparation of polymer-based monolithic reactors are as follows.
1. Copolymerization of more than two monomers in the presence of porogens (Fréchet-type monoliths) [21–26].
2. Preparation of diblock copolymers in which a well-defined cylindrical and degradable polymer is embedded inside a second polymer followed by selective removal of the degradable polymer [27].
3. Polymerization of a monolithic polymeric phase wedged inside the microchannel pore system of an inert support such as glass and other preformed inorganic materials [14].
The third concept, the PASSflow (polymer-assisted solution phase synthesis) technique, was originally developed by Kunz and Kirshning and has been utilized in a variety of heterogeneous reactions under both batch and flow conditions. The monolith consists of a chemically functionalized and highly porous polymer–glass composite. This monolith was synthesized by precipitation polymerization of styrene and a functionalized vinylic monomer with 2,2′-azobis(2-methylpropionitrile) (AIBN) within porous glass rods. For example, functionalized monoliths bearing a quaternary ammonium moiety were prepared by treatment of copolymer that was prepared from vinylbenzyl chloride with styrene, with triethylamine. Various monoliths bearing a functional group such as sulfonic acid, amine, pyridine, and imidazole have been reported. As will be shown later, these materials can be utilized in the continuous-flow reactions.
7.2.3 Miscellaneous
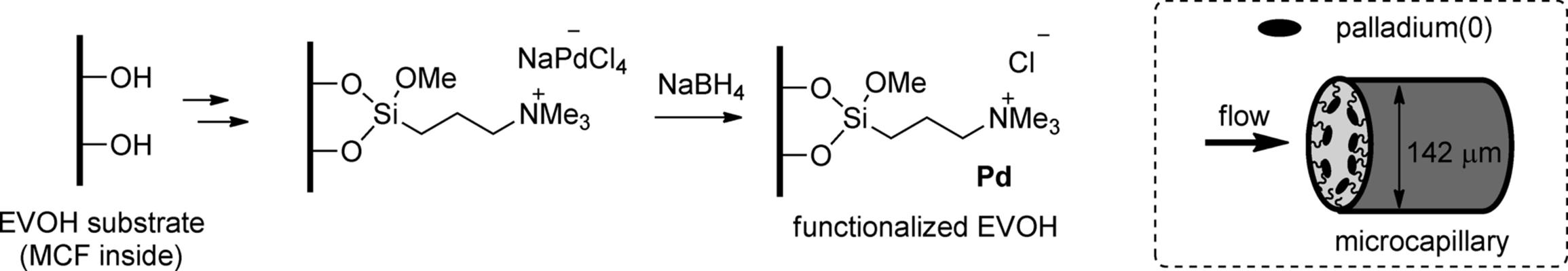
Macklay, Ley, and coworkers reported a different process for the preparation of a flow multi-channel microreactor in which the internal wall was coated by palladium catalyst. The disc-shaped plastic micoreactors, termed microcapillary films (MCFs), contain 19 parallel microchannels, each with a mean internal diameter of 142 ± 10 μm. The material was prepared using a melt extrusion process from an ethylene-vinyl alcohol copolymer (EVOH) [28]. Immobilization of palladium(0) on the wall surface inside the MCFs was performed by simple chemical deposition techniques (Scheme 7.3). The palladium-coated capillaries were used for transfer hydrogenation of ketones, imines, nitro compounds, alkenes, and alkynes with triethylsilane under flow conditions [29]. Microcapillaries whose inside surfaces were coated with copper or gold were also utilized for the continuous-flow reactions [30].
Scheme 7.3 Wall surface functionalization of microcapillaries (EVOH-MCF) inside and schematic illustration of a wall-coated microcapillary.
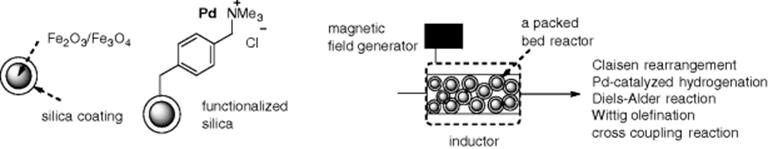
Recently, magnetic nanoparticles have received attention as a new class of solid supports. Such particles, which consist of magnetic elements such as iron and nickel compounds, can be easily removed from a reaction mixture by applying an external magnetic field. In addition, the nanoparticles offer inductive heating of the reaction mixture in the presence of an electromagnetic field. Kirschning et al. reported continuous-flow reactions using a flow packed-bed reactor that was filled with magnetic nanoparticles (Fe2O3/Fe3O4) coated with silica gel (Scheme 7.4) [31, 32]. Their results suggested that inductive heating can be compared with microwave heating with respect to rate acceleration. They utilized functionalized silica-coated Fe2O3/Fe3O4 nanoparticles ligated to Pd(0) in flow hydrogenation and cross-coupling reactions.
Scheme 7.4 Unfunctionalized and functionalized magnetic nanoparticles, and a schematic illustration of the inductive heating-assisted microreactor.
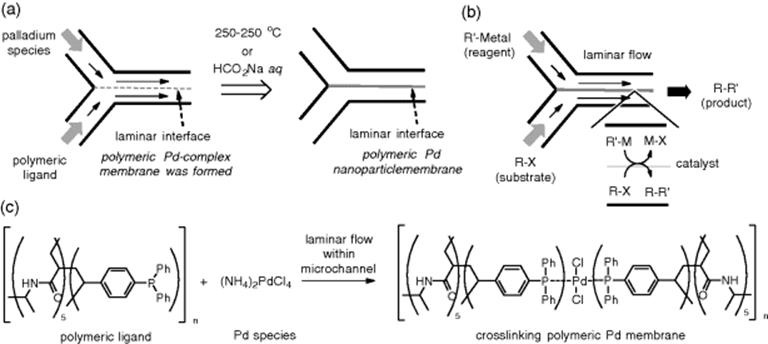
The polymeric catalytic membrane is utilized for biphasic laminar flow synthesis. Uozumi and coworkers developed membrane-installed microflow devices, in which a self-assembled polymeric Pd nanoparticle membrane was installed, for several important chemical transformations. The polymer membrane was precipitated at the laminar interface between the two parallel flows (Scheme 7.5). There is no doubt that the vast interfacial surface of the catalytic membrane with substrates and reactants in the biphasic flows would improve the rate of chemical transformation. And in fact, Suzuki–Miyaura coupling [33], allylic arylation [34], and hydrodehalogenation [35] were achieved with significant efficiency by using the microflow devices with Pd-nanoparticle membranes installed.
Scheme 7.5 (a) Preparation of polymeric Pd-nanoparticle membrane-installed devices, (b) a catalytic biphasic reaction under laminar flow conditions, (c) Pd-cross-linking formation as a catalytic membrane.