Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
7. Heterogeneous Reactions
7.3. Flow Reactions with an Immobilized Stoichiometric Reagent
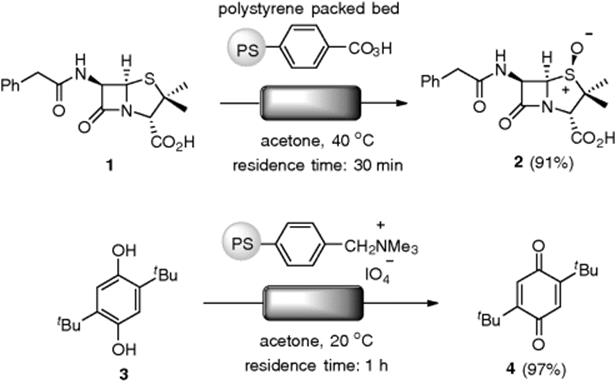
In 1976, Harrison and Hodge reported on the flow oxidation of sulfide using a gravity-fed-type packed-bed reactor consisting of peracid-functionalized polystyrenes. When a solution of penicillin G (1) was allowed to pass once through the column over 30 min (residence time) at 40 °C, the corresponding (S)-sulfoxide (2) was obtained in 91% yield (Scheme 7.6) [36]. This represents an improvement over the yield of 85% for a normal batch experiment for 2 h at 20 °C with a 1.5 equiv. of the resin. The resin could be regenerated to 99% of its previous efficiency, but irreversible degradation of the polymer was observed after several cycles. They also demonstrated an oxidation of hydroquinone (3) into quinone (4) with polymer-supported periodate in the flow system [37]. Polystyrene-based amine polymers, such as PS-TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene), were utilized as a stoichiometric Br![]() nsted base reagent, in the flow alkylation of thiols and sulfonamides [38, 39]. The flow method was applied to the combinatorial synthesis of a compound library.
nsted base reagent, in the flow alkylation of thiols and sulfonamides [38, 39]. The flow method was applied to the combinatorial synthesis of a compound library.
Scheme 7.6 Flow oxidation using packed-bed reactors.
When the Jones oxidation of benzyl alcohol was carried out in a batch reactor, formation of a mixture of the starting material, benzaldehyde, and benzoic acid was observed. In contrast, the flow conditions using a gravity fed-type packed-bed reactor filled with the silica-supported Jones reagent revealed very unique results. While only benzaldehyde was isolated in quantitative yield at the fast flow rate (650 μl/min), slower passing (50 μl/min) of the substrate resulted in quantitative conversion into benzoic acid. Unfortunately, leaching of chromium reagent from the silica support was also observed in this system [40].
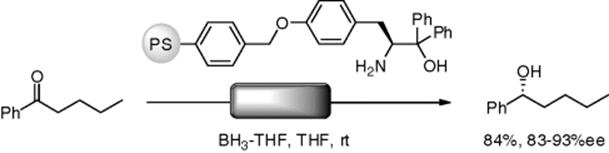
Istuno and coworkers reported an enantioselective reduction of ketones using a flow reactor, in which polymer-supported amino alcohol was fixed (Scheme 7.7). As butyl phenyl ketone and borane (1.2 equiv.) in THF were separately and continuously pumped into the reactor (overflow type), the desired alcohol was obtained in 84% yield with 83–93% ee. With analogous batch reactions, ee values were 81%–92%. Thus, with this reaction, both the flow and batch procedures gave similar results [41].
Scheme 7.7 Enantioselective reduction under the flow conditions.
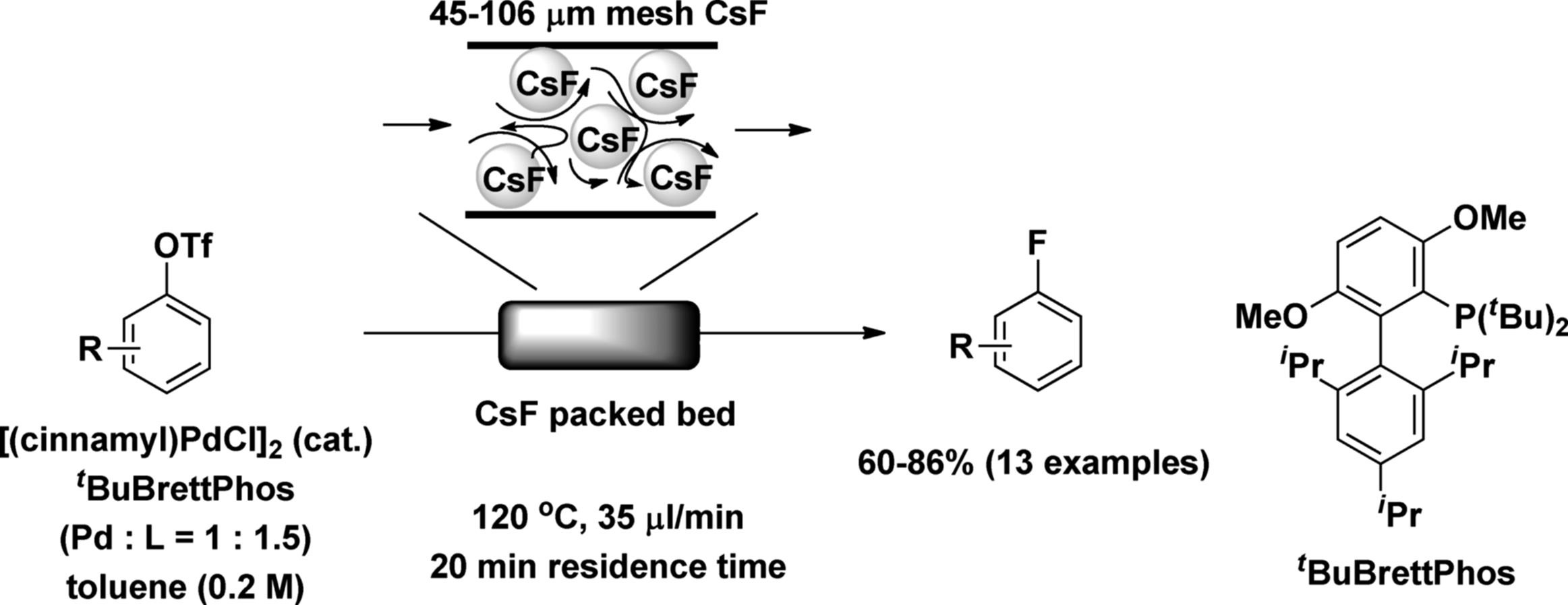
A solid reagent itself, such as a metal, metal oxide, or metal salt, can be fixed in a packed-bed reactor. Buchwald and coworkers developed a CsF packed-bed reactor for the Pd-catalyzed conversion of aryl triflates to aryl fluorides in flow. It is very important to achieve a uniform flow distribution in the packed bed. To this end, they prepared a microreactor filled with finely ground CsF that had been filtered to obtain a uniform particle size distribution of approximately 45–106 μm. By using the packed-bed reactor, a variety of (hetero)aryltriflates were converted into the corresponding aryl fluorides in the presence of a catalytic amount of homogeneous Pd(0) catalyst within short reaction times under flow conditions (Scheme 7.8). The resulting reaction rate was faster than in the batch reaction. This would have been due to the mixing efficiency and higher CsF surface-to-volume ratio. The method allows easy handling of large quantities of insoluble CsF with precise control [42].
Scheme 7.8 Palladium-catalyzed CߝF bond formation under the flow conditions.
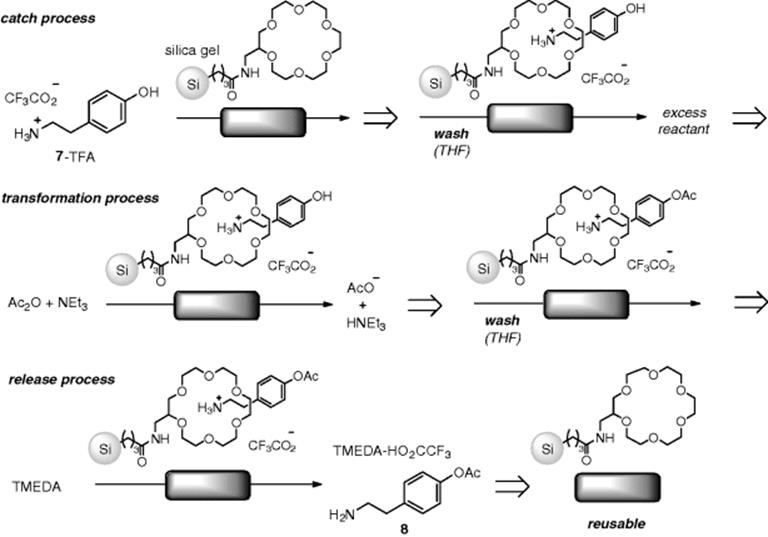
As an interesting example of a stoichiometric reaction in flow, Watts reported a protecting group-free synthesis by a flow reactor approach. When the reaction was performed in the absence of the protecting group, formation of a mixture of amide, ester (8), and diacetate was observed. On the other hand, their previously developed, immobilized 18-crown-6 on silica gel, coupled with a flow technique, enabled a selective O-acylation of tyramine (7) without amide formation. Their proposed reaction sequence is summarized in Scheme 7.9 [43]. First, ammonium was selectively protected by the 18-crown-6 moiety of the polymer based on a host–guest interaction (step a). Selective O-acetylation occurred with the assistance of a weak base (step b). Washing with tetramethylenediamine (TMEDA) resulted in deprotection of 8 and crown ether regeneration (step c). Though the study was just at the research level, this “catch and release” strategy could have an impact at the industrial level as well [44, 45].
Scheme 7.9 Protecting group-free flow synthesis: selective O-acylation of tyramine (7) based on a “catch and release” concept.
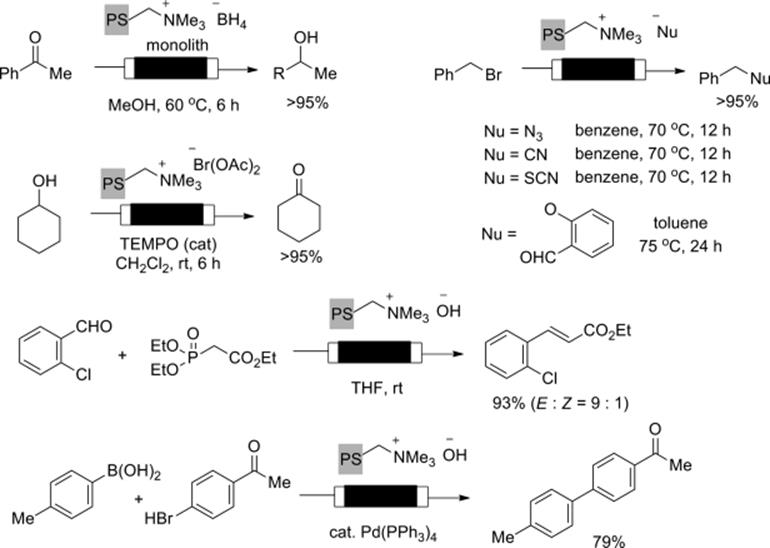
Stoichiometric reagents immobilized on a monolith also enhanced the efficiency of flow chemical transformations. Kirschning and Kunz prepared polymer–glass monoliths incorporating a reagent moiety, which facilitate various reactions such as reduction, reductive amination, oxidation desilylation, and alkylation to be efficiently performed in the flow-through mode (Scheme 7.10) [46]. Horner–Wadworth–Emmons olefinations [47] and Suzuki–Miyaura couplings [48, 49] were achieved based on the same concept. They also developed polymer-bound bisazido iodate for the synthesis of vinyl azides starting from the corresponding alkenes in the flow mode. Furthermore, they demonstrated multistep flow synthesis of triazoles by the combination with copper-catalyzed Huisgen cycloaddition under the flow conditions [50].
Scheme 7.10 Stoichiometric reactions using flow monolith reactors.
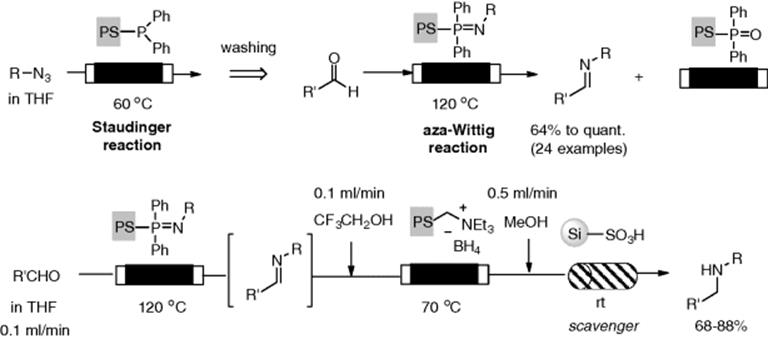
Several monolithic supports to facilitate key chemical transformations have been investigated. Ley et al. developed an azide monolithic reactor for conducting Curtius rearrangement [51]. His group developed a monolithic phosphine as a useful reactor, which was prepared by precipitation polymerization of the appropriate phosphine monomer (Figure 7.2). The reactor facilitated a Staudinger aza-Wittig reaction sequence under flow conditions based on a catch and release concept (Scheme 7.11) [52]. The Staudinger reaction was carried out by passing a solution of aryl or alkyl azide through the monolith with heating at 60 °C to give immobilized iminophosphorane. Then, an aza-Wittig reaction was employed by loading an aldehyde or ketone at 120 °C through the reactor, which resulted in production of the corresponding imine. Though preparation of a clean immobilized iminophosphorane intermediate was required, only simple washing with the appropriate solvent allowed removal of any impurities to provide a clean intermediate on the support. When the flow reactors consisting of ammonium borohydride and an appropriate scavenger column were attached, the multistep flow synthesis of amines from azides and aldehydes could be carried out.
Figure 7.2 Monolithic phosphine reactors, with and without a glass column housing (7 cm length × 10 mm i.d.). Source: From ref [51].

Scheme 7.11 Staudinger-aza-Wittig reaction in a catch and release reactor and its application to multistep flow synthesis.
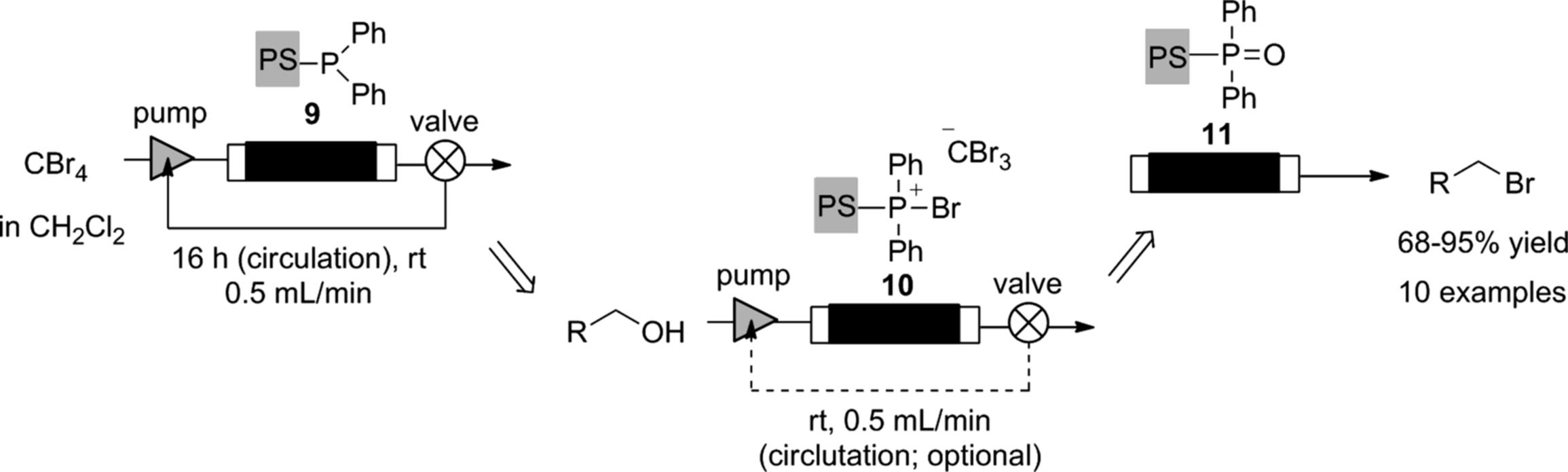
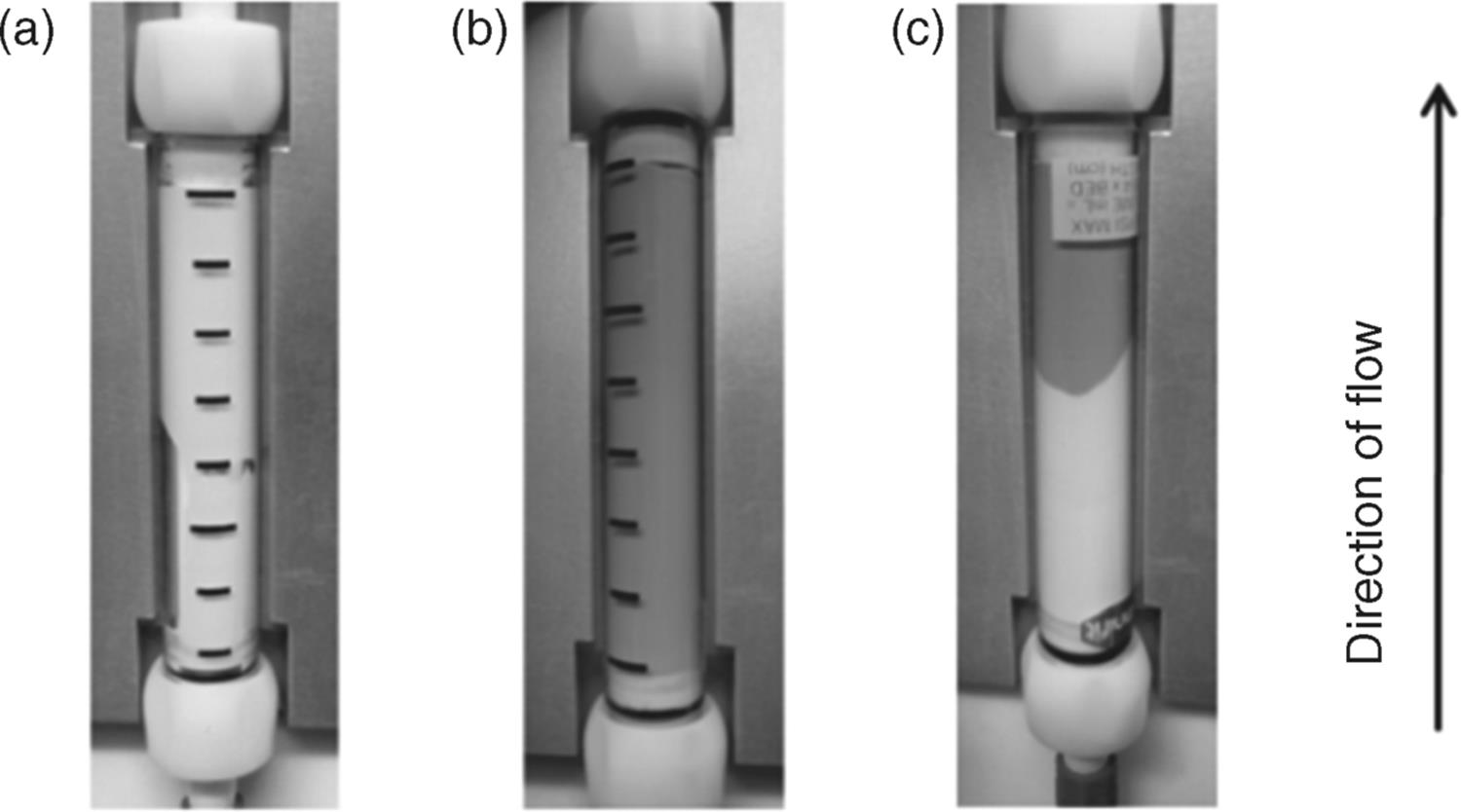
The “catch and release” monolithic phosphine can be employed in a flow Appel reaction [53]. The monolith (9) was treated with carbon tetrabromide to give the active bromophosphonium (10), with which alcohols were transformed into alkyl bromides. As a result, the functional group on the monolith changed into phosphine oxide. Although complete bromination of reactive benzyl alcohols was achieved by a single pass of the alcohol through the monolith, it was found to be necessary to recycle the flow stream through the monolith to obtain complete conversion for less-activated substrates (Scheme 7.12). Interestingly, the flow reaction can be monitored by the color change of the monolith column (Figure 7.3). The original color of the phosphine-functionalized monolith (9) was white (a). As the bromophosphonium (10) formed, the monolith turned brown (b). Treatment of (10) with an alcohol resulted in a further color change of the monolith from brown to white (c), with a pale yellow region corresponding to the phosphine oxide (11). A similar color-changing monolith, which was functionalized with N-(tert-butyl)phenylsulfinimidoyl chloride, was also developed and evaluated for the flow oxidation of alcohols and amines by the same author [54].
Figure 7.3 (a) Unfunctionalized triphenylphosphine monolith; (b) monolith after functionalization with carbon tetrabromide; (c) monolith after partial consumption of the active brominating agent. Source: From ref [53].
Scheme 7.12 Flow transformation of alcohols into alkyl bromides.