Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
7. Heterogeneous Reactions
7.7. Organocatalysis in Flow Reactions
In recent years, the development of environmentally benign processes for chemical synthesis has attracted a growing interest. Organocatalysis offers several advantages because it is generally non-toxic and can tolerate the presence of moisture [147]. However, organocatalytic reactions often require high catalyst loading and a long reaction time for the achievement of high conversion. A microreactor system using immobilization of organocatalysts allows higher turnover numbers (>TONs) of the catalytic reactions and simple recovery of the catalysts [148].
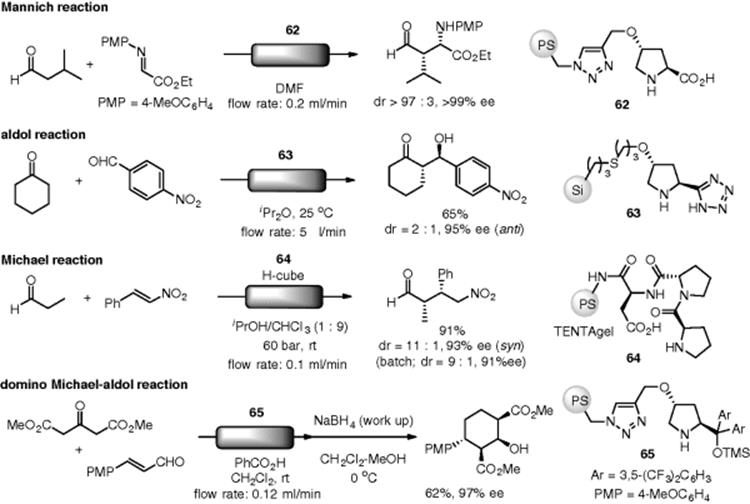
The use of supported organocatalysts in flow chemistry is not new. A pioneering work using an organic base catalyst was reported by Venturello. Knoevenagel condensations of aromatic aldehydes, cyclohexanone, and acetophenone with acetoacetate, cyanoacetate, or malonate were catalyzed by aminopropyl-functionalized silica gel (56), which was packed in a gravity-fed column, under continuous-flow conditions (Scheme 7.40) [149]. A flowcell microreactor, whose wall surfaces were coated with aminopropylsilica, was utilized in Knoevenagel and Michael reactions [150].
Scheme 7.40 Flow Knoevenagel condensation catalyzed by polymer-supported amine.
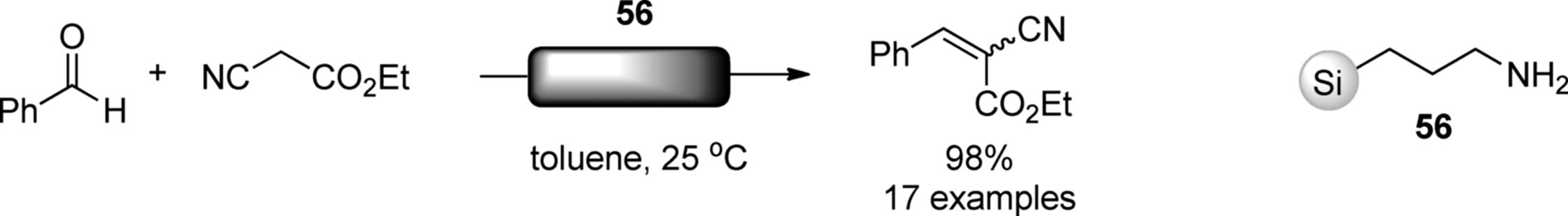
Several polymer-supported amine catalysts were reported for continuous-flow reactions [151]. Ley and coworkers achieved a continuous-flow synthesis of 4,5-disubstituted oxazoles [152]. Their flow system consisted of two microreactors and one scavenger column, as shown in Scheme 7.41. Isocyanine and acid chloride were mixed on a glass-tip microreactor to give a reactive intermediate. Progressing this combined reaction stream through a packed column of polymer-supported phosphazene base (57) (PS-BEMP) facilitated an intramolecular cyclization yielding the substituted oxazoles. The excess acid chloride was removed by a scavenger column, Quadrapure-BZA. The flow system could generate an oxazole library in high yields and high purities without an additional purification. The flow system can be an excellent platform for synthesis of compound libraries [153, 154]. McQuade and coworkers compared the catalytic activities of immobilized organocatalysts under batch and flow conditions. Triazabicyclodecene (TBD) and dimethylaminopyridine (DMAP) analogs bound to methacrylated-based resins (58) and (59) were employed in Knoevenagel condensation and acylation of alcohol, respectively. Compared with batch reactors, both (57) and (58) showed three- to six-fold increased productivity owing to the improved mixing and decreased dimensions of the channel [155]. TEMPO (2,2,6,6-tetramethylpiperidine1-oxyl) catalyzes an oxidation of alcohols into ketones or aldehydes. The same group prepared polymethacrylate-supported TEMPO for a flow oxidation and evaluated its efficacy by using a biphasic plug flow reactor [156].
Scheme 7.41 Flow synthesis of 4,5-disubstituted ozaxoles.
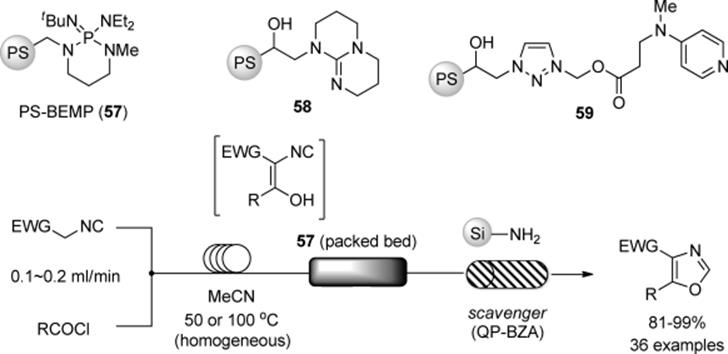
When organocatalysts are chiral, an avenue is opened to asymmetric catalysis. In 2001, Lectka and coworkers reported a continuous-flow synthesis of β-lactams using a polymer-supported chiral organocatalyst [157]. Among their designed flow systems was the in-series of three gravity-fed columns shown in Scheme 7.42. The respective columns were filled with PS-BEMP (57) for ketene formation, quinine immobilized on Wang resin (60) for achieving [2 + 2]-cycloadditions, and an amino-scavenger (61) for the removal of ketene and acid chloride. It has to be noted that such reaction cascades can only be used when each of the reactions proceeds at a similar rate.
Scheme 7.42 Flow β-lactam synthesis from acid chloride and imine.
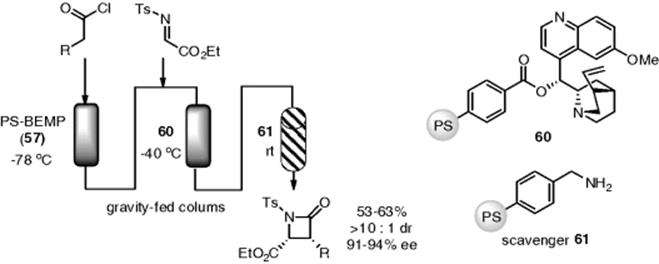
Since the discovery of proline-catalyzed enantioselective aldol reactions, an extensive research program to explore chiral secondary amine catalysts has been pursued. Several polymer-supported chiral amines have been synthesized for aldol, Mannich, and related reactions. Polystyrene is a popular solid phase for use in place of silica gel in the proline-based organocatalysis. In contrast, silica gel displays a slightly acidic character and has a hydrogen-bond donor or acceptor, which may change the catalytic activity and chiral space of the organocatalyst. Flow enantioselective aldol [158–161], Mannich [162], Michael [163], and related reactions [164, 165], using polystyrene-supported chiral secondary amine catalysts, have been reported by several researchers (Scheme 7.43). Asymmetric chlorinations and Michael reactions in flow were achieved by using a quinine-based polymer catalyst [166, 167].
Scheme 7.43 Enantioselective aldol and related reactions under flow conditions.