Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
7. Heterogeneous Reactions
7.8. Flow Biotransformation Reactions Catalyzed by Immobilized Enzymes
Solid-supported enzymes for use in batch reactions are well-known, and the exploitation of biotransformation reactions under flow conditions has been the focus of much research. Herein, only a few representative examples of enzymatic catalysis in microreactors are described [168–171].
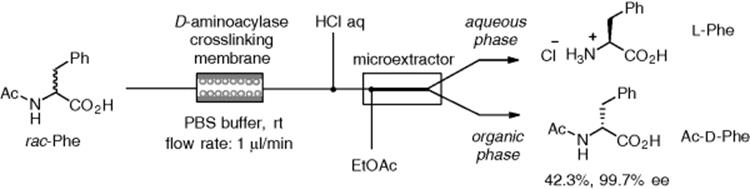
Miyazaki and Maeda accomplished immobilization of acylase by the formation of an enzyme-polymeric membrane on the inner wall of the microreactor [172]. The same group used a microreactor system connected to a microextractor, which allowed liquid–liquid microextraction in a flow stream, as shown in Scheme 7.44. Using this microreaction system, optical resolution of racemic acetylphenylalanine was achieved to give d-acetyl phenylalanine with high optical purity [173].
Scheme 7.44 Kinetic resolution of dl-acetyl phenylalanine.
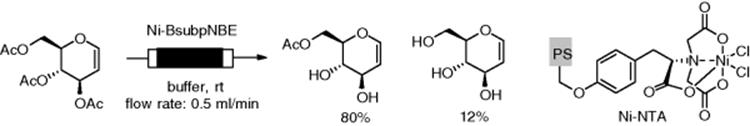
Dräger and Kirschning and coworkers synthesized a tyrosine-based Ni-nitrilotriacetic acid (Ni-NTA) linker system attached to a monolith for immobilization of enzymes. The Ni-NTA motif displays high affinity to the hexahistidine sequence in proteins (His6-tag), giving a heterogeneous complex. The specific interaction was utilized to prepare polymer-supported enzymes. p-Nitrobenzyl esterase from Bacillus subtilis (BsubpNBE) was immobilized on the Ni-NTA monolith and was used to study several biotransformations. Deacetylation of tri-O-acetyl-d-glucal was achieved under flow conditions to give 6-O-acetyl-d-glucal with a good regioselectivity (Scheme 7.45) [174].
Scheme 7.45 Flow-through deacetylation of triacetyl-d-glucal.