Microreactors in Organic Chemistry and Catalysis, Second Edition (2013)
8. Liquid–Liquid Biphasic Reactions
8.7. Micromixer
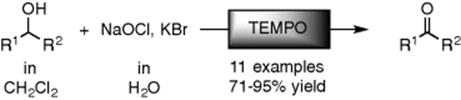
Biphasic catalysis conducted in fluorous media has attracted attention as the interest in environment-friendly synthetic processes is growing. The advantages of microsystems for biphasic fluorous catalysis were investigated by studying the Mukaiyama aldol reaction to form a carbon–carbon bonds between aldehydes and silyl enol ethers in the presence of a Lewis acid catalyst [22] (Figure 8.11). The reaction was conducted in a biphasic fluorous/organic solvent system using a low concentration of the lanthanide complex Sc[N(SO2C8F17)2]3 as a catalyst. The catalyst dissolves only in the fluorous phase, while the substrate and product are soluble in the organic phase. A borosilicate microdevice was used and the delivery of reagents was controlled by a precise syringe delivery system. Both phases were delivered through a Y-junction at high pressure. After the reaction, the product was easily isolated from the organic phase while the catalyst remained in the fluorous phase demonstrating the biphasic advantage of the product and catalyst separation.
Figure 8.11 Mukaiyama Aldol reaction in a borosilicate microreactor under parallel flow. Source: By courtesy of Elsevier [22].
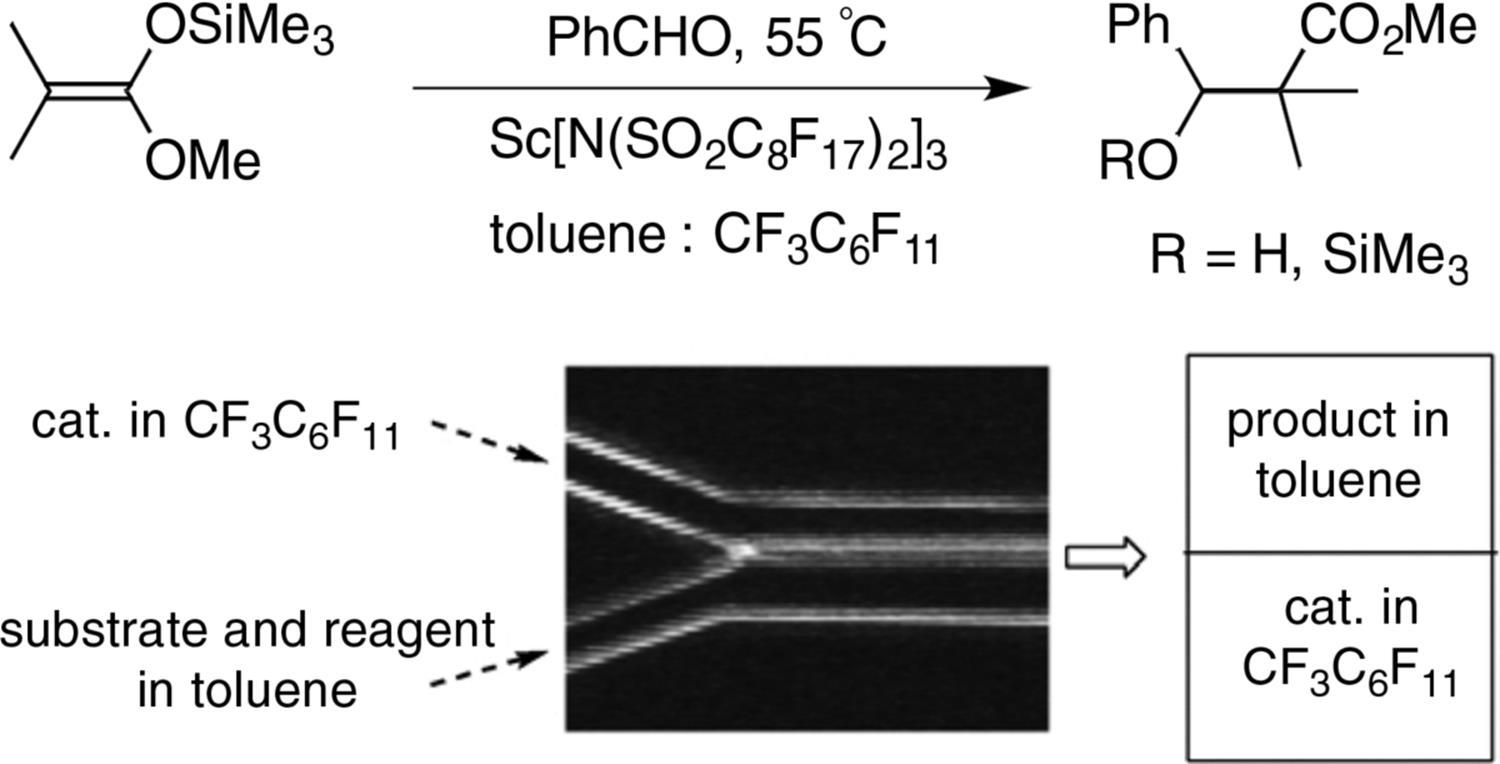
Another efficient aldol condensation has been reported by Fukase using deprotonated acetone as the enolate [23]. The yields reported are superior to the corresponding batch reactions. Mikami et al. [24] were able to increase the reaction rate of a Sc[N(SO2C8F17)2]3 catalyzed Baeyer–Villiger reaction (Figure 8.12). Only one regioisomeric lactone was obtained in high yields even at very low catalyst concentrations (0.05 mol%).
Figure 8.12 Baeyer–Villiger reaction in a microreactor. Source: By courtesy of Elsevier [24].
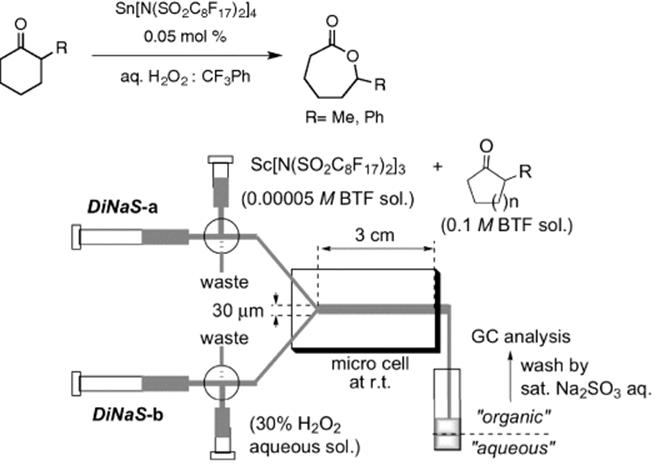
Conducting a reaction under parallel flow in microreactor system was also demonstrated by Kitamori et al., where high conversions were achieved for phase-transfer catalyzed reactions [25]. Using a glass microchip, the reaction of p-nitrobenzene diazonium tetrafluoroborate in water and in ethyl acetate took place in the aqueous layer after rapid phase transfer of 5-methylresorcinol used in excess (Scheme 8.3). Not only higher yields compared to a conventional reaction in a flask were observed due to the large specific interfacial area in the microreactor but also no side products could be detected due to fast removal of the main product from the aqueous to the organic phase. The separation of the two phases was easily achieved by splitting the reaction channel into two channels at the end.
Scheme 8.3 Reaction of 5-methylresorcinol with p-nitrobenzene diazonium tetrafluoroborate.
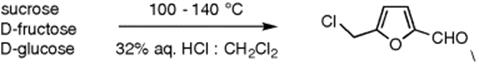
The dehydration of carbohydrates to furfural derivatives has been investigated by Tsanaktsidis et al. [26] (Scheme 8.4). The carbohydrates such as sucrose, d-fructose, and d-glucose were dissolved in the aqueous phase of 32% hydrochloric acid and mixed at a T-piece with the organic phase. Defined organic segments were formed before entering the reactor coil where the solution was heated to temperatures ranging from 100 to 140 °C. Reaction times in the minutes range resulted in yields of up to 81%. A similar procedure had been achieved earlier by Loebbecke et al., where the dehydration of fructose to 5-hydroxymethylfurfural was reported [27].
Scheme 8.4 Dehydration of carbohydrates to 5-(chloromethyl)furfural under biphasic conditions.
The use of phase-transfer catalysts in biphasic microflow systems has grown largely in the last few years [28–30]. Phase-transfer catalysts are used to allow reactants in two immiscible phases to undergo a more efficient reaction by allowing insoluble materials to pass into their originally immiscible solvent. This has been used extensively in batch conditions for many years and been shown to increase productivity in industrial applications such as polymerization of polyesters. Coupling this technique with microreactor chemistry has also been shown in many recent publications.
Ahmed-Omer et al. [31] have shown that phase-transfer catalyzed segmented flow in a capillary microreactor can even be accelerated by sonication. The hydrolysis of p-nitrophenyl acetate in toluene with 0.5 M aqueous sodium hydroxide at different temperatures was used as a test reaction for this system (Scheme 8.5). Sonication and tetrabutylammonium hydrogen sulfate as phase-transfer catalyst were used to increase the rate of reaction showing an increase in the yields. It was also shown in a comparison of flow forms that segmented flow had an advantage over parallel flow because of the increased surface to volume ratio. The reaction in flow was also shown to be superior to batch conditions. A more in-depth investigation of the effect of sonication on the hydrolysis of p-nitrophenyl acetate has been performed by Jänisch and Hübner et al. [32]. They showed that sonication of the reaction was indeed beneficial showing increased yields compared to silent conditions.
Scheme 8.5 Hydrolysis of p-nitrophenyl acetate with aqueous sodium hydroxide in the presence of a phase-transfer catalyst.
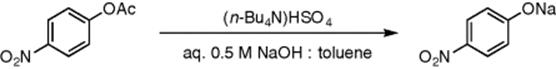
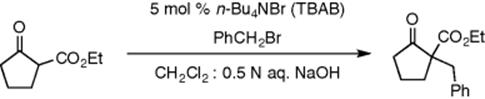
Benzylations reaction using TBAB (tetrabutylammonium bromide) as a phase-transfer catalyst in glass microchannel reactors have been investigated as shown in Scheme 8.6 [33]. A dichloromethane/aqueous biphasic system was used in which both the substrate (ethyl 2-oxocyclopentanecarboxylate) and the alkylating agent (benzyl bromide) are dissolved in the organic phase, while the transfer catalyst TBAB is dissolved in the NaOH aqueous phase. However, in this case the geometry of the microreactor induced the formation of segmented flow as visualized using an optical microscope. Studies on the effect of the microchannel size on the alkylation reaction were performed. It was found that smaller channels in the microreactor lead to higher rates as the interfacial area increases.
Scheme 8.6 Phase transfer alkylation of ethyl 2-oxocyclopentane carboxylate with benzyl bromide in the presence of TBAB.
The synthesis of tert-butyl peroxypivalate from pivaloyl chloride and deprotonated tert-butyl peroxide has been investigated using segmented flow [34]. The small interfacial tension between the two phases caused problems in this process, which was solved using higher dilutions to achieve a regular droplet formation.
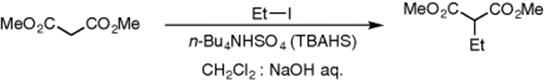
Another example of phase-transfer catalysis in biphasic reactions performed in microreactors was reported by Okamoto [35]. Segmented flow was employed using alternating pumping in order to increase the yield of a biphasic alkylation reaction of malonic ester with iodoethane in the presence of the phase-transfer catalyst TBAHS (tetrabutylammonium hydrogensulfate) (Scheme 8.7, Figure 8.13). Alternate pumping is a technique used to create segmented flow in a microchannel without having to consider the geometry of the inlet junction or the properties of the liquids involved. More detailed studies concerning the principles of this technique without application to chemical reactions have been published by various authors [36–38].
Figure 8.13 Alternate pumping of solution A, containing iodoethane and dimethyl malonate in dichloromethane, and solution B containing TBAHS in a solution of aq. sodium hydroxide. Source: By courtesy of Wiley-VCH Verlag GmbH [35].
Scheme 8.7 Alkylation of malonic ester with iodoethane in the presence of TBAHS as a phase-transfer catalyst.
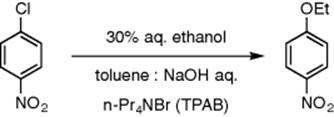
Phase-transfer catalysis has also been used by Alibour et al. in the generation of a tri-phasic system for the ethoxylation of p-chloronitrobenzene in a single channel microchip reactor using tetrapropyl ammonium bromide (TPAB) as the phase-transfer catalyst [39] (Scheme 8.8). The two initial phases consist of an organic phase with p-chloronitrobenzene in toluene and an aqueous phase of TPAB in 30% aqueous ethanol. In the microreactor, parallel flow is induced and a third catalyst-rich phase between the organic and aqueous layer is formed. This is generated because TPAB has limited solubility in the organic and aqueous phases above critical concentration levels. A comparison of reaction rates between batch and microflow was conducted showing a large increase in efficiency for the flow system. The same research group investigated the reaction between disodium sulfide and benzyl chloride using tetrahexyl ammonium bromide as a phase-transfer catalyst. The reaction was also assisted by sonication [40]. Sonication was used to accelerate the hydrolysis of benzyl cloride to benzyl alcohol in a biphasic system [41]. Temperature effects have also been investigated.
Scheme 8.8 Ethoxylation of p-chloronitrobenzene with aqueous sodium hydroxide in the presence of TPAB.
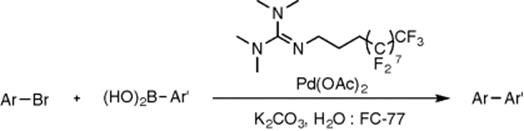
An interesting approach into biphasic systems has been undertaken by Huck et al. [42]. These authors have used a system of aqueous microdroplets in a catalytically active fluorous phase (FC-77) for Suzuki–Miyaura coupling reactions. A fluorous-tagged palladium catalyst is constantly recycled in the fluorous solution using a peristaltic pump in a circular PTFE tubing reactor. The reacted aqueous phase is separated using a separating column and new substrate is fed in initiating the cycle again with the recycled catalyst. The catalyst head group is soluble in the aqueous phase, while the tail is only soluble within the fluorous phase, therefore the catalysis happens at the phase boundary of the microdroplets. Aryl bromides and boronic acids were coupled at room temperature with varying residence times showing good to excellent yields (Scheme 8.9).
Scheme 8.9 Coupling of aryl bromides and boronic acids with palladium in the presence of a fluorous tagged guanidine ligand in a fluorous–aqueous biphasic system.
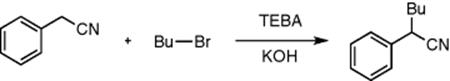
Segmented flow and phase-transfer catalysis was employed by Jovanovic et al. [43] to increase the selectivity and productivity of the selective alkylation of phenylacetonitrile (Scheme 8.10). The reaction system consisted of an organic phase containing n-butyl bromide and phenylacetonitrile and an aqueous phase of sodium hydroxide and triethylbenzyl ammonium chloride (TEBA). The segment size was analyzed as a function of productivity where the smaller segments increased the conversion from 40% (465 μm slugs) to 99% (240 μm slugs) as the surface to volume ratio was increased. Although the conversion was increased, the selectivity was decreased as a dialkylation occurred, therefore, an intermediate size of slug was chosen to give the best compromise where the conversion was maintained at 74% with 99% selectivity. When compared with batch, this is an increase in conversion by 1.8 times and a 12% increase in selectivity.
Scheme 8.10 The alkylation of phenylacetonitrile with butyl bromide and potassium hydroxide in the presence of TEBA.
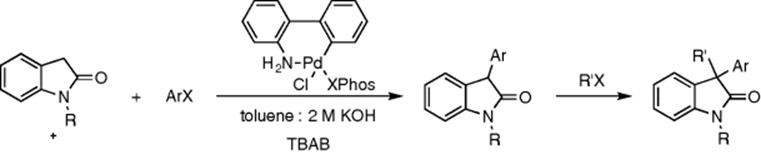
The synthesis of 3,3-disubstituted 2-oxindoles by palladium-catalyzed α-arylation/alkylation sequences in a biphasic flow system has been achieved by Buchwald et al. [44]. The reaction sequence consisted of two steps, the arylation followed by the alkylation. The first reaction system contains the substrate and aryl halide in toluene in one solution, the second the pre-catalyst in toluene, and a third solution containing potassium hydroxide and tetrabutyl ammonium bromide (TBAB) (Scheme 8.11). This is then mixed in a packed-bed mixer and heated to 100 °C for arylation. This system is then fed into a second T-piece where the alkyl halide is introduced, further mixing is applied in the second packed-bed reactor for the alkylation. This product mixture is then quenched with monosodium phosphate and extracted with ethyl acetate. The overall reaction proceeded in a 93% yield and could also be stopped at the arylation step by the addition of a switch valve before the alkylation step. Palladium-catalyzed hydrogenations have also been investigated under organic/aqueous biphasic conditions and an enhancement has been observed when using polymeric encapsulation [45].
Scheme 8.11 The arylation followed by alkylation of 2-oxindoles in a toluene:potassium hydroxide biphasic system catalyzed by a palladium precatalyst and a phase-transfer catalyst TBAB.
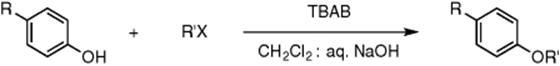
Phase-transfer catalysis is clearly very powerful in biphasic microreactors as shown in the various examples earlier. The same system has also been applied in flow for the O-alkylation of phenols using alkyl halides in a sodium hydroxide/dichloromethane biphasic mixture in a PTFE microreactor (Scheme 8.12). TBAB was again used as the phase-transfer catalyst and the reaction mixture heated at 90–100 °C. A range of phenols and alkyl halides showed very good yields within 2.5–10 min of reaction time. These results were compared with conventional heating methods showing much improved conversion rates and microwave irradiation where the conversion was almost as efficient [46]. Similar work has been done by Yang et al. using again TBAB in a microdroplet system. The synthesized benzyl phenyl ethers were used to test this system [47].
Scheme 8.12 The O-alkylation of phenols using alkyl halides in a sodium hydroxide/dichloromethane biphasic media using TBAB as phase-transfer catalyst.
The industrially important nitration of aromatic compounds in a microreactor using two immiscible liquid phases was demonstrated in different studies using either parallel [48] or segmented flow [49]. In all studies, a PTFE capillary microchannel, connected to an inlet junction, was used in which either segmented or parallel flow can be created. The use of PTFE tubing is desirable as it is commercially available and no complicated microfabrication methods are involved.
In the macroscale reaction, the formation of side products such as dinitrobenzene and picric acid is expected as a result of mass transfer limitations. Hence, using a microreactor system, the formation of the side product was reduced. And the rate of reaction was increased.
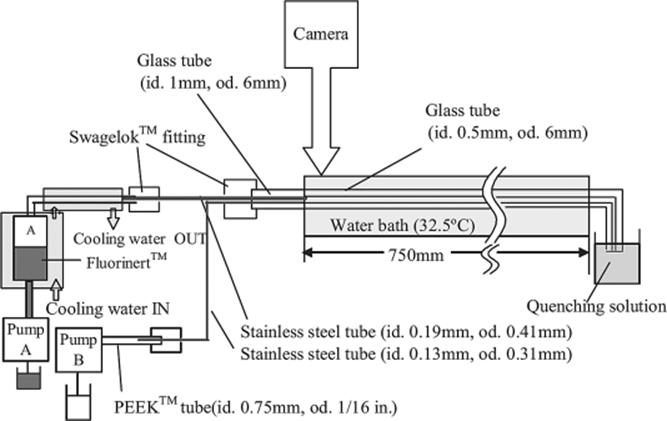
Single-phase reactions can also be conducted in segmented flow manner in which the reactions take place within a segment, while the other immiscible phase is used to form segments. This is a useful way to generate regular turbulence in laminar flow in single-phase reactions [50]. Ismagilov et al. [51] demonstrated this technique to optimize deacetylation of ouabain hexa acetate as an example of reaction systems in the microchannel. To do that, they have developed a screening system shown in Figure 8.14a, in which each segment contains a different reagent separated by a fluorinated carrier fluid while taking an advantage of an optical detection with MALDI-MS.
Figure 8.14 (a) Flow screening system for deacetylation of ouabain hexaacetate (R1–R6 = Ac) reaction optimizations. (b) After incubation, the segments were deposited onto a sample plate for MALDI-MS. Source: By courtesy of the American Chemical Society [51].
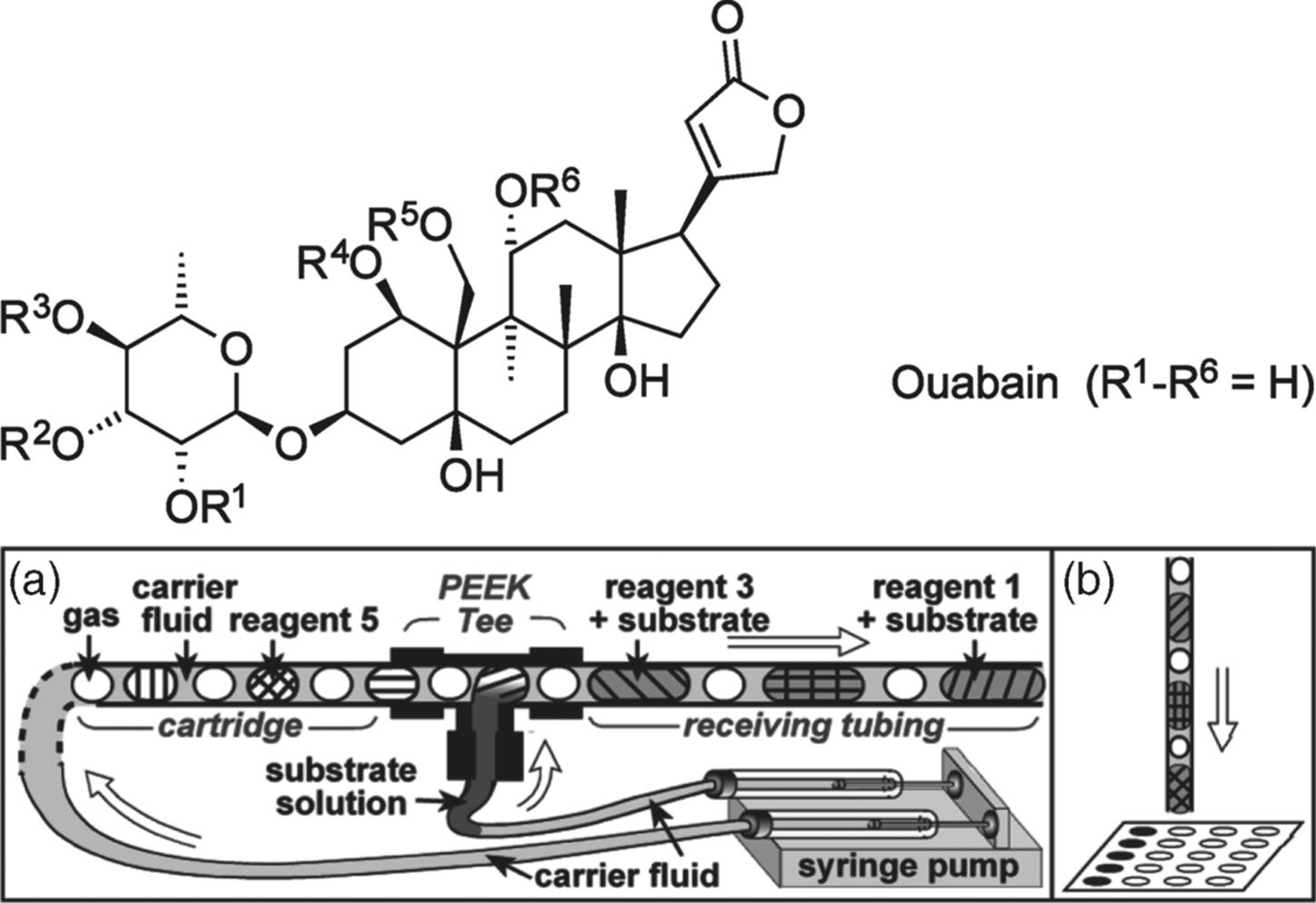
The segments were introduced in sequence to the carrier fluid flow where they combine with the substrate solution. A blank solvent segment was introduced between the reagent segments to avoid contamination between them [52]. Following that the flow of the segments was stopped and the microtube system was sealed to keep the segments inside for a specified reaction time. Then, the segments were released, collected, and analyzed.
The same concept, originally developed by Ismagilov [50], was applied to homogeneous catalyzed reactions by introducing an immiscible solvent to the flow generating segmented flow instead of a single flow. As demonstrated by Wirth et al. [53], the reaction yield was enhanced for various Heck products compared to conventional methods, and further improving the outcome when using the segmented flow instead of the single flow [54].
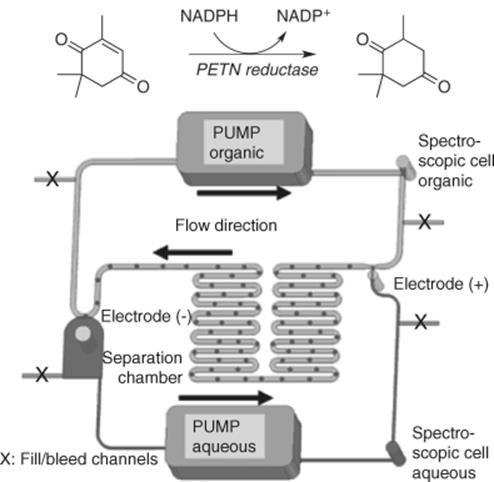
Also biocatalytic reactions have been shown to be facilitated by biphasic flow systems (Scheme 8.13). Esterifications in ionic liquid/heptane mixtures [55] as well as hydrolysis of esters in water/decane solvents [56] are accelerated in biphasic flow systems due to efficient product removal from the enzymes used in these transformations. Also reductions of carbon–carbon double bonds using pentaerythritol tetranitrate (PETN) reductase have been investigated in a recycling organic/aqueous biphasic flow system as shown in Figure 8.15 using ketoisophorone as a substrate [57]. The stoichiometric oxidant is NAD(P)+ and the reaction solutions are monitored through two integrated spectroscopic cells. Electrodes are also included in the device to facilitate a fast phase separation.
Figure 8.15 Biocatalytic reductions in a recirculating biphasic flow system MS. Source: By courtesy of The Royal Society of Chemistry [57].
Scheme 8.13 Heck-reactions accelerated by biphasic flow system.
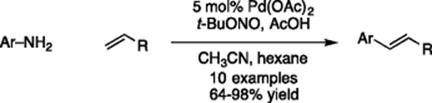
Further biocatalytic studies have been performed using segmented biphasic flow by Schmid et al. [58]. An enzymatic catalyzed reduction of 1-heptaldehyde to 1-heptanol with the organic phase consisting of 1-heptaldehyde in hexadecane and bis–tris buffer, NADH, TADH, FDH, and ammonium formate in the aqueous phase was investigated. They used this system to demonstrate a way of analyzing the method of flow chemistry for enzyme catalyzed reactions and whether the increased mass transfer from this method is a benefit to a specific reaction. In doing so, an “operation window” was established where they consider an effectiveness factor, the Damköhler number, and the productivity to determine the benefit in a flow regime.
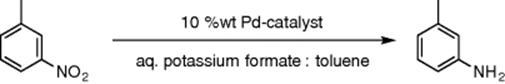
Chemical reductions have also been investigated. Ufer et al. [59] have produced a suspended catalyst in a two-phase slug system by adapting the wetting properties to the specific phase. The internal vortex mixing within the solvent slugs was determined to be an important factor in keeping the activity of suspended catalyst high. The catalytic transfer hydrogenation of m-nitrotoluene in an organic phase of toluene containing nitrotoluene with 10 wt% of suspended palladium on carbon particles to m-toluidine with an aqueous potassium formate solution was chosen for this investigation. The reaction was carried out at 70 °C (Scheme 8.14). After hydrolysis of the reaction mixture, the catalyst was filtered off from the aqueous phase and the product isolated. The effect of the flow rate on the internal vortex mixing and also the addition of an inert gas was studied.
Scheme 8.14 Hydrogenation of m-nitrotoluene to m-toluidine with potassium formate using a palladium catalyst.
Segmented flow together with an immobilized reagent in a packed-bed reactor has been used by McQuade and Bogdan in an alcohol oxidation protocol [60]. The plugs of aqueous sodium hypochlorite as oxidant and the alcohol dissolved in dichloromethane are merged into an emulsion on the packed-bed microreactor containing an immobilized TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) catalyst. The oxidation to the carbonyl compound is very efficient and the large slugs leaving the microreactor separate the organic product from the aqueous by-products as shown in Scheme 8.15.
Scheme 8.15 Biphasic oxidation in a packed-bed microreactor.