Why Is Milk White?: & 200 Other Curious Chemistry Questions (2013)
Preface
I was talking one day with my coauthor, 11-year-old Alexa Coelho, about science, in particular about chemistry, which is her favorite subject. I was explaining that in much of science, the hard part is coming up with the right questions. Once you ask the right questions, half the work is done.
She had been reading a book that had two authors. One was a person with a special story to tell, and the other was a person who had written several books and knew how to put the right words onto paper to tell the story well. Alexa thought it might be fun to write a book but thought she would need some assistance from someone who knew the ins and outs of the publishing business. I agreed to help her in her endeavors.
Alexa then took the reins and worked very hard, without any assistance, doing the hard part of science. She spent almost all of her free time coming up with page after page of questions about chemistry that she wished she knew the answers to.
When she had come up with an astounding 200 questions about chemistry, she made me a gracious and generous offer. She would split the profits from her book 50/50 with me, if I would do the easy part and write the answers to the questions. I accepted her kind offer, and the result is this book.
Along with the addition of a few fun projects and a glossary, the questions got trimmed a bit, as happens during editing. But Alexa assures me that all of the important ones are still there.
How to Read
Structural Formulas
Throughout this book, you’ll see drawings that illustrate a particular chemical’s structural formula—how it’s shaped at a molecular level. Knowing its shape is often quite useful in understanding how it behaves and how it interacts with other compounds.
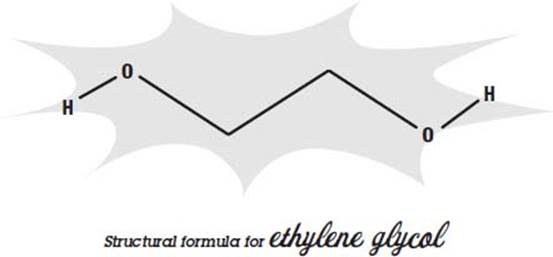
Chemists use a few simplifying rules to show how a molecule is shaped without cluttering up the picture. Most of the atoms that make up a molecule are labeled; in the structural formula above, hydrogen is labeled with an “H” and oxygen is labeled with an “O.” But carbon atoms are so common that they are not labeled with a “C.” Instead, they are assumed to be anywhere on a formula where two lines join, like the two middle bends in the above drawing.
And since hydrogens attached to carbons are also very common, and carbon always has four bonds, any place on a formula where fewer than four lines join, it is assumed that hydrogens fill the carbon’s remaining bonds, and so they are not labeled either. That means that there are two more hydrogen atoms attached to each of the unlabeled carbons at the center of the formula on the previous page.