Why Is Milk White?: & 200 Other Curious Chemistry Questions (2013)
8. Chemistry in the World
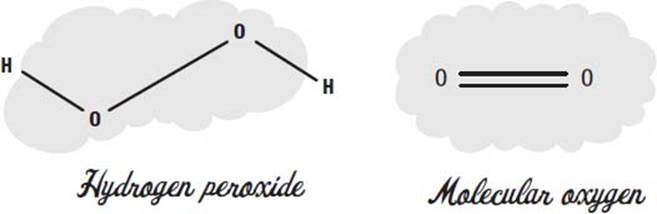
What is hydrogen peroxide?
Hydrogen peroxide is two atoms of oxygen, each attached to a hydrogen atom. You can think of it as a water molecule with an extra oxygen in the middle.
The oxygen we breathe has two oxygen atoms in it—we call it molecular oxygen. In molecular oxygen, the two atoms share two electrons, in what we call a double bond.
When two oxygens are connected with a single bond, we call it a peroxide. Hydrogen peroxide is the simplest example of a peroxide molecule.
Pure hydrogen peroxide is a liquid a little heavier and thicker (more viscous) than water. It is a strong oxidizer and can start fires if it contacts flammable materials like wood or paper.
In the home, dilute solutions of hydrogen peroxide are used to disinfect and bleach. Three percent hydrogen peroxide is used to clean wounds and as a mouth rinse to kill bacteria. Stronger solutions are used to bleach hair.
Cells produce hydrogen peroxide as a byproduct of metabolism. But hydrogen peroxide is also used by the immune system as a signal that attracts white blood cells to an infection.
A 3 percent solution of hydrogen peroxide will produce ten times its volume in oxygen if a catalyst (such as blood or dry yeast) is added. Most common metals will also act as a catalyst for the dissociation of hydrogen peroxide into oxygen and water. Adding a catalyst to hydrogen peroxide is thus an easy way to generate oxygen.