Why Is Milk White?: & 200 Other Curious Chemistry Questions (2013)
9. Chemists
PROJECT: COPPER CATALYST
Adult
supervision
required
Materials
Protective goggles
Paper towel
Glass jar or similar glass container
1 tablespoon acetone (available at hardware stores)
1 foot of copper wire (available at hardware stores)
Pencil
Pliers or tongs
Gas kitchen stove
The catalytic converter used in cars with internal combustion engines uses platinum and palladium catalysts to combine unburned fuel with oxygen to produce carbon dioxide and water vapor. This prevents the unburned fuel from creating smog. At the same time, the catalysts break down nitrogen oxides that also contribute to air pollution.
The converter needs to be hot in order to work, but once it starts working, the reaction provides extra heat to keep the catalyst hot. It actually glows red hot in use.
In this project, you are going to perform a similar catalytic reaction, but instead of using expensive noble metals like platinum and palladium, you will use simple copper wire as the catalyst. The drawback to using copper is that it is only able to catalyze a small number of fuels, such as methanol and acetone. But acetone is easy to come by at your local hardware store.
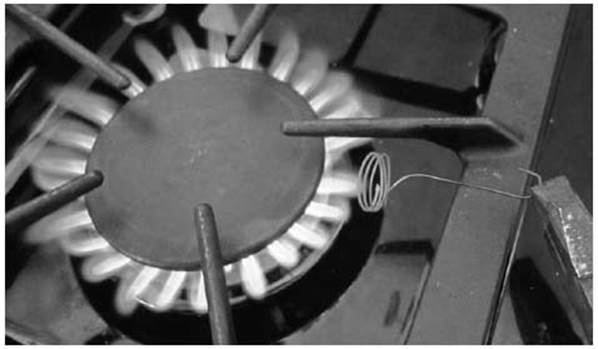
Start by putting on protective goggles. Curl up a strip of paper towel use it to line the sides of a glass jar as shown. Then drop in about a tablespoon of acetone. The paper towel ensures that a lot of acetone vapor stays in the jar. You also need approximately 1 foot of copper wire, bent into a hook on one end and coiled up on the other end as shown, and a pencil from which to hang the wire. Make sure the wire will fit nicely in the jar.
Since the catalyst needs to be hot for the reaction to happen, use pliers or tongs to hold the wire in the flame of a gas stove until it is red hot and glowing.
The last step is to hang the glowing copper coil from the pencil so that the coil hangs in the center of the jar.

The copper coil gets even hotter than it was before we put it in the jar, as the acetone vapor and oxygen combine on the surface of the copper, releasing heat. There is no flame, since the vapor is not actually burning. It is hot, however, and the copper glows yellow and white. You can feel the heat rising from the jar if you put your hand over it.

The reaction will last as long as there is acetone vapor to consume. The tablespoon of acetone I used in this project lasted several minutes, and the coil glowed hot enough to read by in the dark.