Why Is Milk White?: & 200 Other Curious Chemistry Questions (2013)
3. Household Chemistry
PROJECT: PAPER CHROMATOGRAPHY
Materials
Food coloring (yellow, red, and green)
Paper towel
Jar
1 teaspoon salt
2 cups water
Wire coat hanger
Binder clip
¼ cup rubbing alcohol (optional)
¼ cup nail polish remover or acetone (optional)
Chemists have many ways to separate mixtures of compounds into their individual components. But few are as colorful and simple as paper chromatography.
You may have seen or even done paper chromatography, but this project goes into a little more detail than is usually seen in popular books and websites.
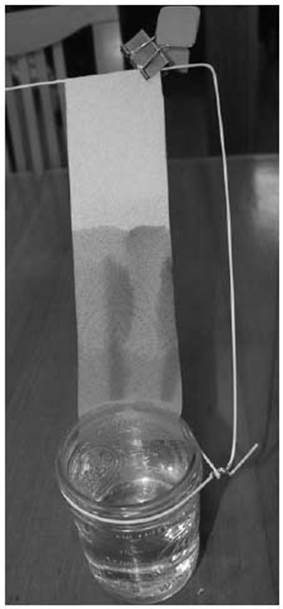
The photo on the next page shows four spots of food coloring on a strip of paper towel: yellow, red, and green, as well as a spot made of all three colors combined. The end of the paper is resting in a jar of 1 percent saltwater solution (1 teaspoon of salt in 2 cups of water). As the salt water wicks up into the towel, it carries some of the dye molecules faster than it does others. Pure water could be used, but the salt water helps make some compounds move faster up the paper than others.

The entire setup is shown on the right. A bit of wire from a coat hanger and a binder clip support the paper towel, and you can see the colors have separated as they climbed up the paper.
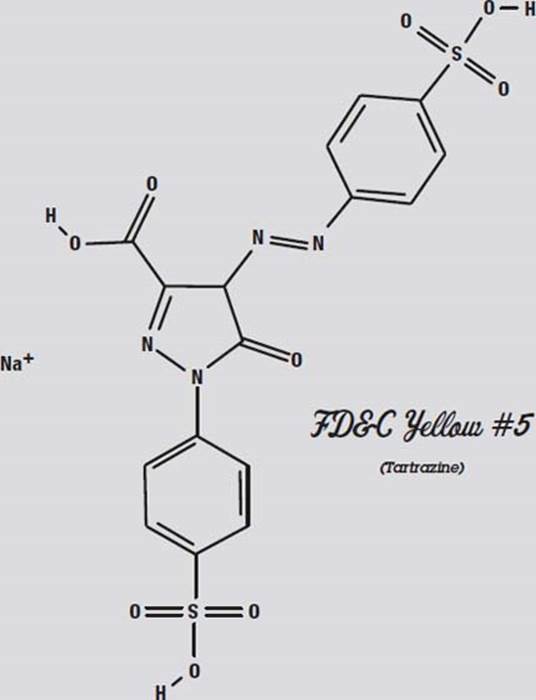
On the next page, the different areas are marked with labels. The box of food coloring that was used says it contains the colors FD&C Yellow #5, FD&C Blue #1, FD&C Red #40, and FD&C Red #3. Notice that there is no green color listed, and there are two different colors of red.
The two reds show up as separate areas of color. The bottom one is a slightly magenta red, and the upper one is a slightly orange red. Combined, they make the color that the dye manufacturer wanted, a rich solid red color.
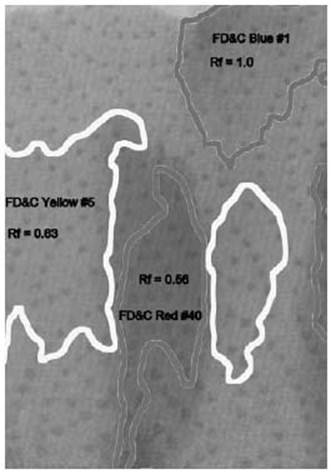
The green food coloring has also separated into two patches of color. The blue color climbed the towel quickly, staying with the very top of the water that wicked up into the towel. Below it is a patch of yellow, still stained faintly with some of the blue, so it appears a little greener than the pure yellow in the first column.
In the last column, three of the four colors have separated. The yellow and one of the reds seem to climb the paper at the same rate, and stay mixed. But the blue raced to the top, and the magenta red lagged behind, barely moving.
The speed of the molecules relative to the salt water is called the retardation factor, or Rf. It is simply the distance the color has moved up the towel divided by the distance the water has moved. We use an estimate of where the center of the color spot is for the calculation.
The paper is called the stationary phase because it stays in place. The salt water is called the mobile phase because it is the part that climbs up the towel. The colors are called the analyte because they are what we are analyzing.
Paper chromatography separates compounds based on how polar they are. A polar molecule has one end that is more positively charged than the other end. A nonpolar molecule does not have charged ends. Paper is made of cellulose, which is a polar molecule. Salt water is also polar, but to a different extent. As the dye molecules encounter the paper and the salt water, some are bound more tightly to the paper, and some more tightly to the water. This causes the separation.
You can take advantage of this effect by using solvents that are less polar than water—for instance, isopropyl alcohol (rubbing alcohol) or acetone (nail polish remover)—to get different separations. With adult supervision,you can experiment with ¼ cup of each one to see what works best for the molecules you are trying to separate.
Using 91 percent isopropyl alcohol, the following chromatogram shows that Red #3 travels faster than the others.
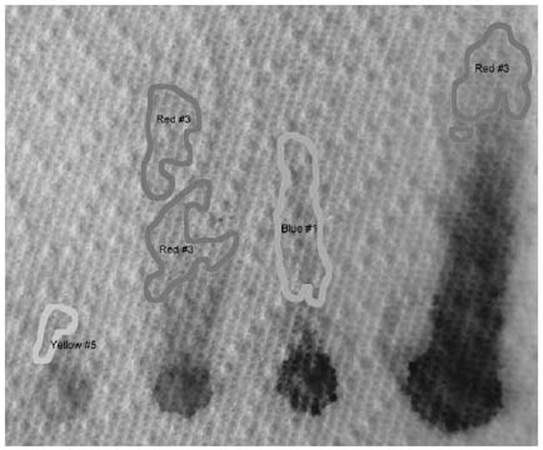
The other colors don’t separate nearly as well as they did in water. In acetone (not shown), only the Red #3 moved. What this shows us is that Red #3 is the least polar of the molecules, and Blue #1 is the most polar.
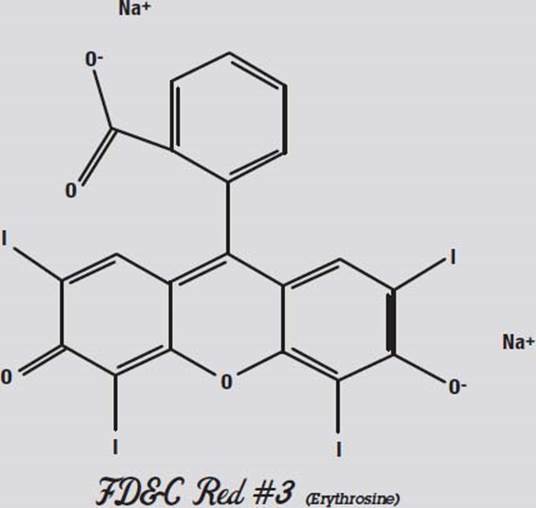
Red #3, shown above, is not very polar. Both ends have negative oxygen atoms attracting positive sodium atoms.
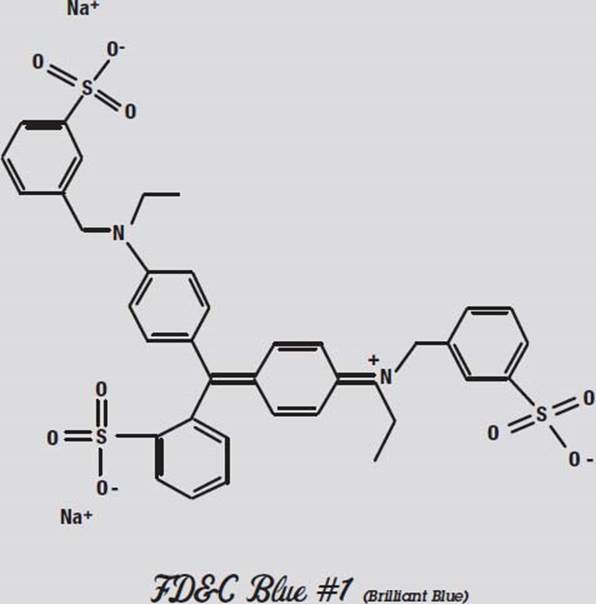
FD&C Blue #1, on the other hand, has one of its negative oxygen atoms alone at the far right, and the compensating positive nitrogen atom is buried towards the middle. This molecule is more polar than the other colors.
Red #40 and Yellow #5 are very similar molecules. That is why they are hard to separate. They react similarly to both the paper and the solvent.
While chromatography was originally invented to separate colored molecules, the technique is so useful that it is now used for a large number of molecules that have no color. To make them visible, chemists can view them in ultraviolet light and look for fluorescence, or they can add a chemical to the developed chromatogram that makes the different spots visible. One such chemical is iodine. Vapors of iodine are allowed to react with the finished chromatogram, and the resulting compounds are often colored.
Other types of chromatography use sensors other than the human eye to distinguish the different molecules. This allows scientists to analyze a huge number of molecules that do not interact with visible light.
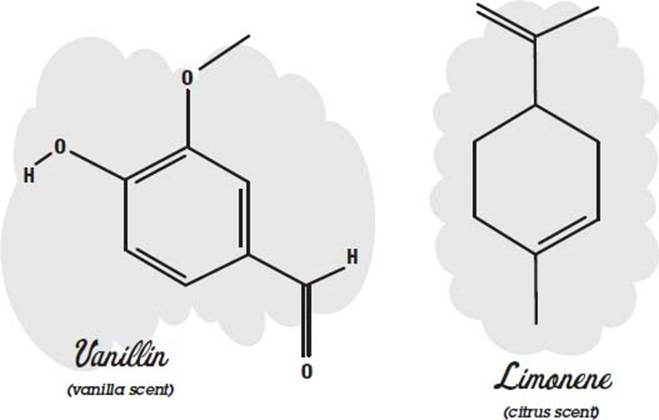
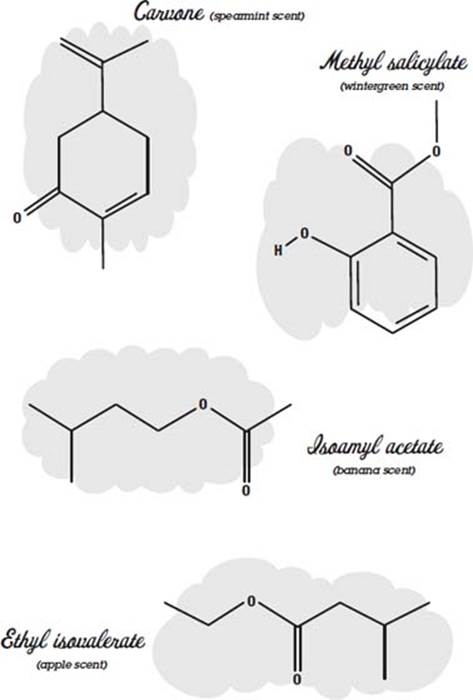
Many perfumes are made of essential oils. Here the word essential does not mean that you can’t do without it but instead refers to the fact that the oil was extracted from a flower, fruit, or some other thing as an essence. This is often done by heating the material until it boils and then collecting and condensing the vapors (distilling). Other methods are solvent extraction, where the material is crushed in a solvent such as alcohol to dissolve the oils, and chromatography, where the oils wick up in a substance and separate out according to how fast they travel.
There may be 300 different compounds in an essential oil. Usually the compounds include one or more molecules that give it its characteristic odor. These molecules can be used by themselves instead of the essential oil to reduce cost or ensure reproducibility and stability in the final product.
Some examples of molecules that have characteristic scents are shown below.