Why Is Milk White?: & 200 Other Curious Chemistry Questions (2013)
3. Household Chemistry
PROJECT: SILICON BOUNCY BALL
Adult
supervision
required
Materials
2 tablespoons sodium silicate solution (available at drugstores)
1 tablespoon rubbing alcohol
Disposable cup
Wooden craft stick or disposable plastic spoon
Warm, running water
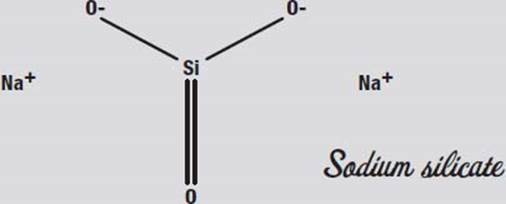
Sodium silicate, also known as water glass or egg keep, is made by reacting quartz with molten sodium carbonate. That is not exactly an easy or safe thing to do at home, however. Another way to make it is to add silica gel (the sandy stuff in those little packets that keep shoe boxes and other packages dry) to a hot solution of lye (sodium hydroxide in water). This is also not exactly a safe thing to play with at home. So it is a good thing that sodium silicate solution is sold in drugstores (although usually behind the counter where you have to ask for it), because sodium silicate is fun to play with.
Sodium silicate got the name egg keep from the practice of coating eggs with it to preserve them before refrigeration was common. It seals the pores in the egg’s shell so that no oxygen can get in. It is also a fireproofing treatment for wood, paper, and cloth for the same reason: oxygen can’t get to the flammable materials.
Those little packets of silica gel mentioned earlier? They are made from sodium silicate. Manufacturers add a little acid, such as hydrochloric acid or even vinegar, to the water glass to make a firm gel. When they dry that gel out in an oven, the water is driven off, and what remains is a delicate network of silicate crystal that has an amazingly large surface area (800 square meters per gram). At the same time the surface attracts water from the air. The result is a very good material to put in packages to protect parts from moist air.
You may have seen “magic garden” kits in toy stores or gift shops. You drop little rocks into a special solution, and the rocks slowly grow into colorful stalagmites that look like little rock trees. The solution they grow in is sodium silicate. The little rocks are made from colorful salts of metals such as copper, cobalt, and manganese.
The bits of copper sulfate or cobalt chloride dissolve and start to react with the sodium silicate, forming insoluble silicate shells around the soluble sulfates or chlorides. But the silicate shells still allow water to get to the crystals inside, and the result is cracks caused by the expansion. The salt solution squirts out, and a new insoluble silicate layer forms around it. This process repeats, forming towers and branches, each with the color of the original salt.
In this project, you are going to use sodium silicate to make a super bouncy ball.
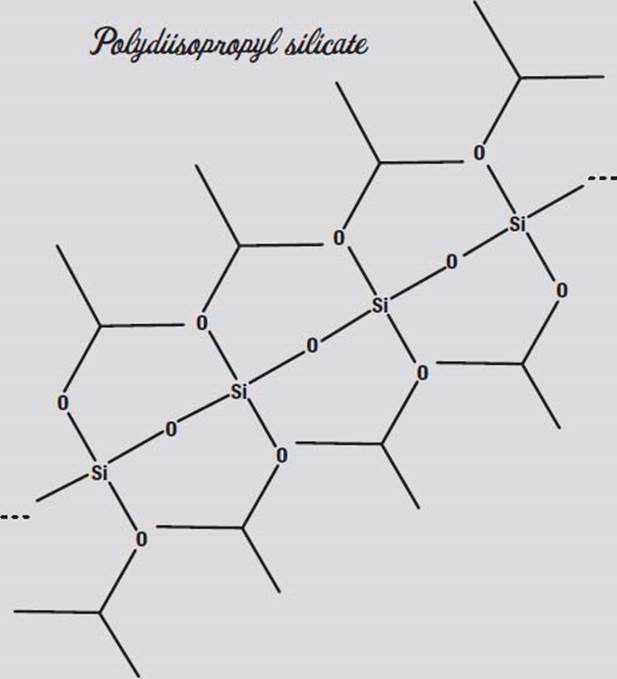
Silicon is right below carbon in the periodic table of the elements, and like carbon, silicon can form polymers (long chains of molecules). You may have used silicone rubber as a glue or sealant. You can make the sodium silicate solution polymerize into long chains by adding an alcohol, such as ethanol or the isopropanol (rubbing alcohol) in your medicine cabinet. The result is polydiethyl or polydiisopropyl silicate.
Place 2 tablespoons of sodium silicate solution into a disposable cup, and add 1 tablespoon of rubbing alcohol.
Next, stir the mixture with a craft stick or disposable plastic spoon for a few seconds to get a thick white mass of polymerized polydiisopropyl silicate, mixed with some water and alcohol.

Now form the mass into a ball with your hands, under running warm water. The warm water helps remove the excess alcohol and form the ball more easily. It also helps wash the slimy polymer from your hands.

The result of our two-minute effort is a remarkably bouncy ball. If dropped, it recovers a large part of the height of the drop on each bounce.