Why Is Milk White?: & 200 Other Curious Chemistry Questions (2013)
3. Household Chemistry
What is in deodorant?
Perfumes in deodorant mask odors. Moisturizers make the skin feel soft and make the product glide onto the skin. Oils are added to make the deodorant more transparent so that it hides better on skin and clothing. Silica is added to absorb skin oils that accumulate from sweat.
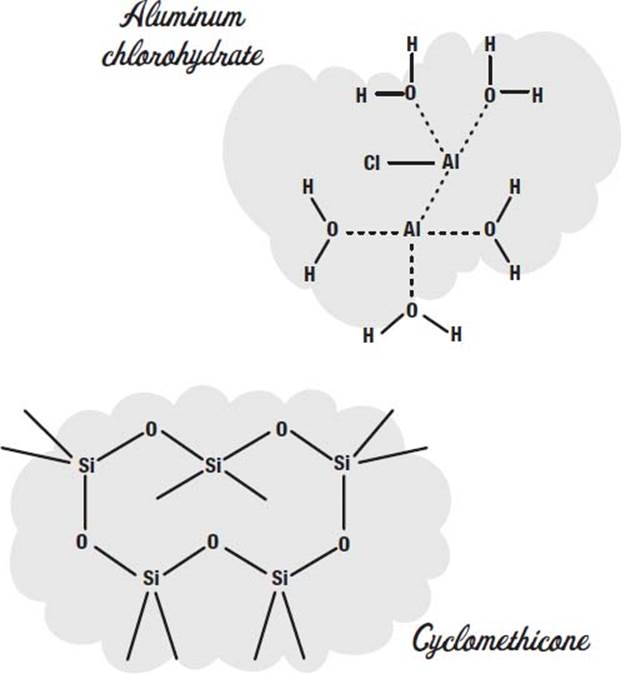
To control perspiration, salts such as aluminum chlorohydrate or aluminum zirconium tetrachlorohydrex GLY are used. These dissolve in the sweat and make a gel that coats the sweat glands.
Aerosol deodorants contain propellants, which are gases under atmospheric pressure but may be liquids in the deodorant can. Butane and isobutane are liquids under pressure or at temperatures you can reach in your freezer. Propane is a gas in the can or in the freezer and is added to the butane to get a higher pressure.
The mix of gases is tailored to the exact pressure needed to propel the other ingredients out of the can without spraying too hard.
In addition to the propellant, a carrier fluid is used to carry the ingredients out in the spray. Cyclomethicone is one common carrier fluid used in aerosol deoderants.
Roll-on deodorants usually have water and alcohol to dissolve the ingredients. The alcohol gives a cooling effect as it dries.
In solid antiperspirants, the ingredients are blended into a solid carrier, which might be a hydrogenated oil or a fatty alcohol like stearyl alcohol.