March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 1. Localized Chemical Bonding
1.H. Dipole Moment
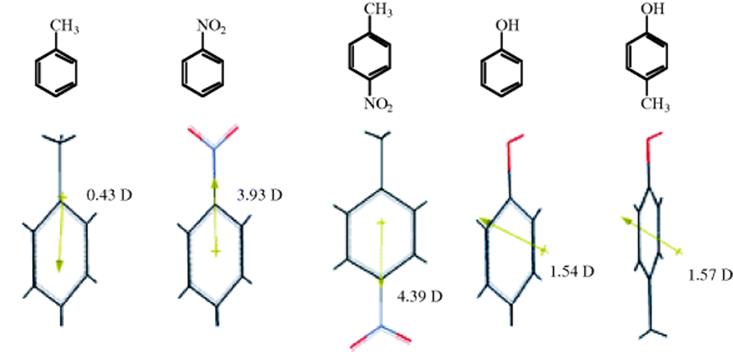
The dipole moment is a property of a molecule that results from charge separations like those discussed above. However, it is not possible to measure the dipole moment of an individual bond within a molecule. Only the total moment of the molecule may be measured, and it is the vectorial sum of the individual bond moments.37 These individual moments are roughly the same from molecule to molecule,38 but this constancy is by no means universal. Thus, from the dipole moments of toluene and nitrobenzene (Fig. 1.11)39 the moment of p-nitrotoluene is predicted to be ~4.36 D. The actual value 4.39 D is reasonable. However, the moment of p-cresol (1.57 D) is quite far from the predicted value of 1.11 D. In some cases, molecules may have substantial individual bond moments, but no total moments at all because the individual moments are canceled out by the overall symmetry of the molecule. Some examples are CCl4, trans-1,2-dibromoethene, and p-dinitrobenzene.
Fig. 1.11 Some dipole moments, in Debye units, measured in benzene. In the 3D model, the arrow indicates the direction of the dipole moment for the molecule, pointing to the negative part of the molecule.39
Because of the small difference between the electronegativities of carbon and hydrogen, alkanes have very small dipole moments, so small that they are difficult to measure. For example, the dipole moment of isobutane is 0.132 D40 and that of propane is 0.085 D.41 Of course, methane and ethane, because of their symmetry, have no dipole moments.42 Few organic molecules have dipole moments > 7 D.