March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 17. Eliminations
17.C. Stereochemistry of the Double Bond
When elimination takes place on a compound of the form CH3–CABX or CHAB–CGGX, the new alkene does not have cis–trans isomerism, but for compounds of the form CHEG–CABX (E and G not H) (24) and CH2E–CABX (25), cis and trans isomers are possible. When the anti E2 mechanism is in operation, 24 gives the isomer
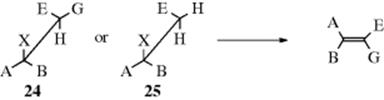
arising from trans orientation of X and H. As seen previously (Sec. 17.A.i), an erythro compound gives the cis alkene and a threo compound gives the trans. For 25, two conformations are possible for the transition state; these lead to different isomers and often both are obtained. However, the one that predominates is often determined by an eclipsing effect.103 For example, Zaitsev elimination from 2-bromopentane can occur as follows: In conformation I, the ethyl group is between Br and Me, while in J it is between Br and H. This means that J is more stable, and most of the elimination should occur from this conformation. This is indeed what happens, and 51% of the trans isomer is formed (with KOEt) compared to 18% of the cis (the rest is the Hofmann product).104 These effects become larger with increasing size of groups A, B, and E.
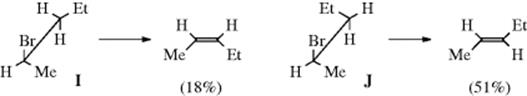
However, eclipsing effects are not the only factors that affect the cis/trans ratio in anti E2 eliminations. Other factors are the nature of the leaving group, the base, the solvent, and the substrate. Not all of these effects are completely understood.105
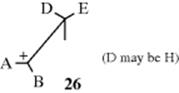
For E1 eliminations, if there is a free carbocation (26), it is free to rotate, and no matter the geometry of the original compound, the more stable situation is the one where the larger of the D–E pair is opposite the smaller of the A–B pair and the corresponding alkene should form. If the carbocation is not completely free, then to that extent, E2-type products are formed. Similar considerations apply in E1cB eliminations.106