March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 18. Rearrangements
18.C. Free Radical Rearrangements54
1,2-Free radical rearrangements are much less common than the nucleophilic type previously considered, for the reasons mentioned in the introductory Section 18.A. Where they do occur, the general pattern is similar. There must first be generation of a free radical, and then the actual migration in which the migrating group moves with one electron:
![]()
Finally, the new free radical will undergo a further reaction to generate a neutral molecule. The order of radical stability leads to a prediction that here too, as with carbocation rearrangements, migrations should be in the order primary → secondary → tertiary, and that the logical place to look for them should be in neopentyl and neophyl systems. The most common way of generating free radicals for the purpose of detection of rearrangements is by decarbonylation of aldehydes (Reaction 14-32). In this manner, it was found that neophyl radicals do undergo rearrangement.55 Thus, PhCMe2CH2CHO treated with di-tert-butyl peroxide gave about equal amounts of the normal product PhCMe2CH3 and the product arising from migration of phenyl56:
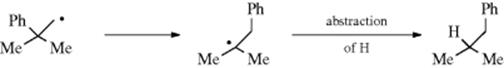
Many other cases of free radical migration of aryl groups have been found.57 Intramolecular radical rearrangements are known.58 The C-4 radicals of α-and β-thujone undergo two distinct rearrangement reactions, and it has been proposed that these could serve as simultaneous but independent radical clocks.59
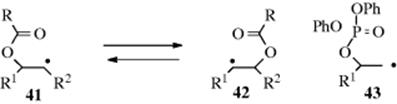
A 1,2-shift has been observed in radicals bearing an OCOR group at the β-carbon where the oxygen group migrates as shown in the interconversion of 41 and 42. This has been proven by 18O isotopic labeling experiments60 and other mechanistic explorations.61 A similar rearrangement was observed with phosphatoxy alkyl radicals (e.g., as 43.62 A 1,2-shift of hydrogen atoms has been observed in aryl radicals.63
Note that the extent of migration is much less than with corresponding carbocations: Thus in the example given, there was only ~50% migration, whereas the carbocation would have given much more. Also noteworthy is that there was no migration of the methyl group. In general, it may be said that free radical migration of alkyl groups does not occur at ordinary temperatures. Many attempts have been made to detect such migration on the traditional neopentyl and bornyl types of substrates. However, alkyl migration is not observed, even in substrates where the corresponding carbocations undergo facile rearrangement.64 Another type of migration that is very common for carbocations, but not observed for free radicals, is 1,2-migration of hydrogen. We confine ourselves to a few examples of the lack of migration of alkyl groups and hydrogen:
1. 3,3-Dimethylpentanal (EtCMe2CH2CHO) gave no rearranged products on decarbonylation.65
2. Addition of RSH to norbornene gave only exo-norbornyl sulfides, though 44 is an intermediate, and the corresponding carbocation cannot be formed without rearrangement.66
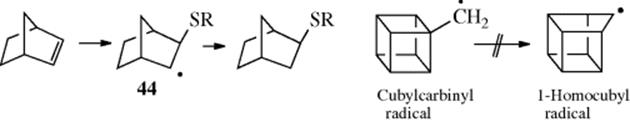
3. The cubylcarbinyl radical did not rearrange to the 1-homocubyl radical, though doing so would result in a considerable decrease in strain.67
4. It was shown68 that no rearrangement of isobutyl radical to tert-butyl radical, which would involve the formation of a more stable radical by a hydrogen shift, took place during the chlorination of isobutane.
However, 1,2-migration of alkyl groups has been shown to occur in certain diradicals.69 For example, the following rearrangement has been established by tritium labeling.70

In this case, the fact that migration of the methyl group leads directly to a compound in which all electrons are paired undoubtedly contributes to the driving force of the reaction.
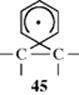
The fact that aryl groups migrate, but alkyl groups and hydrogen generally do not, leads to the proposition that 45, in which the odd electron is not found in the three-membered ring, may be an intermediate. There has been much controversy on this point, but the bulk of the evidence indicates that 45 is a transition state, not an intermediate.71 Among the evidence is the failure to observe 45 either by ESR72 or CIDNP.73 Both of these techniques can detect free radicals with extremely short lifetimes (Sec. 5.C.i).74
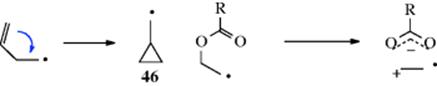
In addition, aryl, vinylic75 and acetoxy groups76 also migrate. Vinylic groups migrate via a cyclopropylcarbinyl radical intermediate (46),77 while the migration of acetoxy groups may involve the charge-separated structure shown.78 Thermal isomerization of 1-(3-butenyl)cyclopropane at 415 °C leads to bicyclo[2.2.1]heptane.79 Migration has been observed for chloro (and to a much lesser extent bromo) groups.
For example, in the reaction of Cl3CCH=CH2 with bromine under the influence of peroxides, the products were 47% Cl3CCHBrCH2Br (the normal addition product) and 53% BrCCl2CHClCH2Br, which arose by rearrangement:

In this particular case, the driving force for the rearrangement is the particular stability of dichloroalkyl free radicals.80 It has been shown that the 1,2-migration of Cl readily occurs if the migration origin is tertiary and the migration terminus is primary.81 Migration of Cl and Br could take place by a transition state in which the odd electron is accommodated in a vacant d orbital of the halogen.
Migratory aptitudes have been measured for the phenyl and vinyl groups, and for three other groups, using the system RCMe2CH2√ → Me2OC√CH2R. These were found to be in the order R = H2C=CH2 > Me3CC=O > Ph > Me3CC![]() C > CN.82
C > CN.82
In summary, 1,2 free radical migrations are much less prevalent than the analogous carbocation processes, and are important only for aryl, vinylic, acetoxy, and halogen migrating groups. The direction of migration is normally toward the more stable radical, but “wrong-way” rearrangements are also known.83
Despite the fact that hydrogen atoms do not migrate 1,2, longer free radical migrations of hydrogen are known.84 The most common are 1,5-shifts, but 1,6 and longer shifts have also been found (see Reaction 18-29). The possibility of 1,3-hydrogen shifts has been much investigated, but it is not certain if any actually occur. If they do they are rare, presumably because the most favorable geometry for C![]() H
H![]() C in the transition state is linear and this geometry cannot be achieved in a 1,3-shift. 1,4-Shifts are definitely known, but are still not very common. These long shifts are best regarded as internal abstractions of hydrogen (for Reactions, see 14-6 and 18-40):
C in the transition state is linear and this geometry cannot be achieved in a 1,3-shift. 1,4-Shifts are definitely known, but are still not very common. These long shifts are best regarded as internal abstractions of hydrogen (for Reactions, see 14-6 and 18-40):
![]()
Transannular shifts of hydrogen atoms have also been observed.85