March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part II. Introduction
Chapter 19. Oxidations and Reductions
First, the terms oxidation and reduction must be clarified. Inorganic chemists define oxidation in two ways: loss of electrons and an increase in oxidation number. In organic chemistry, these definitions, while still technically correct, are not easy to apply. While electrons are directly transferred in some organic oxidations and reductions, and electrons are certainly transferred by making and breaking bonds, the mechanisms of most of these reactions do not involve a direct electron transfer. As for oxidation number, while this is easy to apply in some cases (e.g., the oxidation number of carbon in CH4 is −4), in most cases attempts to apply the concept lead to fractional values or to apparent absurdities. Thus carbon in propane has an oxidation number of −2.67 and in butane of −2.5, although organic chemists seldom think of these two compounds as being in different oxidation states. An improvement could be made by assigning different oxidation states to different carbon atoms in a molecule, depending on what is bonded to them (e.g., the two carbons in acetic acid are obviously in different oxidation states), but for this a whole set of arbitrary assumptions would be required, since the oxidation number of an atom in a molecule is assigned on the basis of the oxidation numbers of the atoms attached to it. There would seem little to be gained by such a procedure. The practice in organic chemistry has been to set up a series of functional groups, in a qualitative way, arranged in order of increasing oxidation state, and then to define oxidation as the conversion of a functional group in a molecule from one category to a higher one. Reduction is the opposite. For the simple functional groups, this series is shown in Table 19.1.1 Note that this classification applies only to a single carbon atom or to two adjacent carbon atoms. Thus 1,3-dichloropropane is in the same oxidation state as chloromethane, but 1,2-dichloropropane is in a higher one. Obviously, such distinctions are somewhat arbitrary, and if we attempt to carry them too far, we will find ourselves painted into a corner. Nevertheless, the basic idea has served organic chemistry well. Note that conversion of any compound to another in the same category is not an oxidation or a reduction. Most oxidations in organic chemistry involve a gain of oxygen and/or a loss of hydrogen (Lavoisier's original definition of oxidation). The reverse is true for reductions.
Table 19.1 Categories of Simple Functional Groups Arranged According to Oxidation Statea
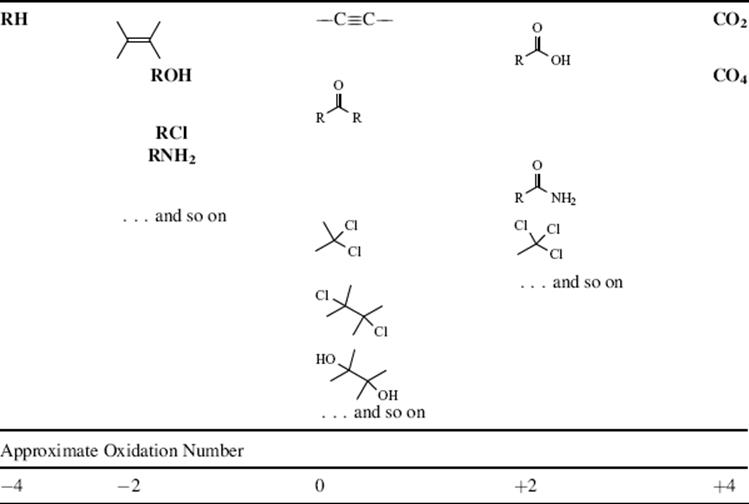
a. Oxidation is the conversion of a functional group in a molecule to a higher category; reduction is conversion to a lower one. Conversions within a category are neither oxidations nor reductions. The numbers given at the bottom are only approximations.
Of course, there is no oxidation without a concurrent reduction. However, reactions are classified as oxidations or reductions depending on whether the organic compound is oxidized or reduced. In some cases, both the oxidant and reductant are organic; those reactions are treated separately at the end of the chapter.
19.A. Mechanisms
Note that our definition of oxidation has nothing to do with mechanism. Thus the conversion of bromomethane to methanol with KOH (Reaction 10-1) and to methane with LiAlH4 (Reaction 19-53) have the same SN2 mechanisms, but one is a reduction (according to our definition) and the other is not. It is impractical to consider the mechanisms of oxidation and reduction reactions in broad categories in this chapter as done for the reactions considered in Chapters 15.2 The main reason is that the mechanisms are too diverse, and this in turn is because the bond changes are too different. For example, in Chapter 15, most reactions involved the bond change C=C → W–C–C–Y yet a relatively few mechanisms covered those reactions. But for oxidations and reductions the bond changes are far more diverse. Another reason is that the mechanism of a given oxidation or reduction reaction can vary greatly with the oxidizing or reducing agent employed. Very often the mechanism has been studied intensively for only one or a few of many possible agents.
Although oxidation and reduction mechanisms are not covered in the same way as other mechanisms, it is still possible to list a few broad mechanistic categories. The scheme of Wiberg is followed.3
1. Direct Electron Transfer.4 Several reactions have been encountered in which the reduction is a direct gain of electrons or the oxidation is a direct loss of them. An example is the Birch reduction (Reaction 15-13), where sodium directly transfers an electron to an aromatic ring. An example from this chapter is found in the bimolecular reduction of ketones with a metal (19-76), where again it is a metal that supplies the electrons. This kind of mechanism is found largely in three types of reaction:5 (a) the oxidation or reduction of a free radical (oxidation to a positive or reduction to a negative ion), (b) the oxidation of a negative ion or the reduction of a positive ion to a comparatively stable free radical, and (c) electrolytic oxidations or reductions (an example is the Kolbe reaction, 14-29). An important example of (b) is oxidation of phenolate ions:
![]()
These reactions occur easily because of the relative stability of the radicals involved.6 The (SET) mechanism, which has been seen several times (see Sec. 10.B) is an important case.
2. Hydride Transfer.7 In some reactions, a hydride ion is transferred to or from the substrate. The reduction of epoxides with LiAlH4 is an example (19-35). Another is the Cannizzaro reaction (19-81). Reactions in which a hydride ion is transferred to a carbocation belong in this category:8
![]()
3. Hydrogen-Atom Transfer. Many oxidation and reduction reactions are free radical substitutions and involve the transfer of a hydrogen atom. For example, one of the two main propagation steps of Reaction 14-1 involves abstraction of hydrogen:
![]()
This is the case for many of the reactions of Chapter 14.
4. Formation of Ester Intermediates. A number of oxidations involve the formation of an ester intermediate (usually of an inorganic acid), and then the cleavage of this intermediate:
![]()
Z is usually CrO3H, MnO3, or a similar inorganic acid moiety. One example of this mechanism will be seen in Reaction 19-23, where A was an alkyl or aryl group, B was OH, and Z was CrO3H. Another is the oxidation of a secondary alcohol to a ketone (Reaction 19-3), where A and B are alkyl or aryl groups and Z is also CrO3H. In the lead tetraacetate oxidation of glycols (Reaction 19-7), the mechanism also follows this pattern, but the positive leaving group is carbon instead of hydrogen. Note that the cleavage shown is an example of an E2 elimination.
5. Displacement Mechanisms. In these reactions, the organic substrate uses its electrons to cause displacement on an electrophilic oxidizing agent. One example is the addition of bromine to an alkene (Reaction 15-39).

An example from this chapter is found in Reaction 19-29:
![]()
6. Addition–Elimination Mechanisms. In the reaction between α,β-unsaturated ketones and alkaline peroxide (15-50), the oxidizing agent adds to the substrate and then part of it is lost:
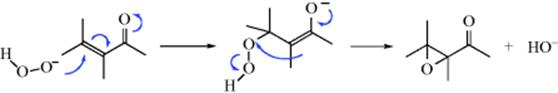
In this case, the oxygen of the oxidizing agent is in oxidation state −1 and the hydroxide ion departs with its oxygen in the −2 state, so it is reduced and the substrate is oxidized. There are several reactions that follow this pattern of addition of an oxidizing agent and the loss of part of the agent, usually in a different oxidation state. Another example is the oxidation of ketones with SeO2 (Reaction 19-17). This reaction is also an example of category 4, since it involves formation and E2 cleavage of an ester. This example shows that these six categories are not mutually exclusive.