March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 2. Delocalized Chemical Bonding
2.B. Bond Energies and Distances in Compounds Containing Delocalized Bonds
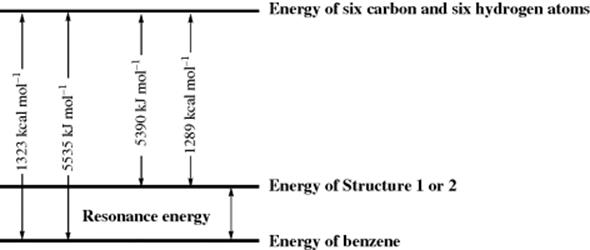
If the energies of all the bonds in benzene are added, taking the values from a source like Table 1.7, the value for the heat of atomization is less than the experimentally determined value (Fig. 2.3) of 1323 kcal mol−1 (5535 kJ mol−1). If E values for a C=C double bond obtained from cyclohexene (148.8 kcal mol−1; 622.6 kJ mol−1) are used, a C–C single bond from cyclohexane (81.8 kcal mol−1, 342 kJ mol−1), and C–H bonds from methane (99.5 kcal mol−1, 416 kJ mol−1), a value of 1289 kcal mol−1 (5390 kJ mol−1) is obtained for structure 1 or 2. By this calculation, the resonance energy is 34 kcal mol−1 (145 kJ mol−1). Of course, this is an arbitrary calculation since, in addition to the fact that a heat of atomization is calculated for a nonexistent structure (1), E values must be used that do not have a firm basis in reality. The actual C–H bond energy for benzene has been measured to be 113.5 ± 0.5 kcal mol−1 at 300 K and estimated to be 112.0 ± 0.6 kcal mol−1 (469 kJ mol−1) at 0 K.26 The heat of atomization of a real molecule can be measured, but resonance energy can never be measured, only estimated, because only an intelligent guess can be made at that of the Lewis structure of lowest energy.
Fig. 2.3 Resonance energy in benzene.
Another method frequently used for estimation of resonance energy involves measurements of heats of hydrogenation.27 The heat of hydrogenation of cyclohexene is 28.6 kcal mol−1 (120 kJ mol−1), so a hypothetical 1 or 2 with three double bonds is expected to have a heat of hydrogenation of ~ 85.8 kcal mol−1 (360 kJ mol−1). Benzene has a measured heat of hydrogenation of 49.8 kcal mol−1 (208 kJ mol−1), and the difference between the measured and expected value is the resonance energy, 36 kcal mol−1 (152 kJ mol−1). By any calculation, the real molecule is more stable than a hypothetical 1 or 2.
The energies of the six benzene orbitals can be calculated from HMO theory in terms of two quantities that are labeled α and β. Here α is the amount of energy possessed by an isolated 2p orbital before overlap, while β (called the resonance integral) is an energy unit expressing the degree of stabilization resulting from π-orbital overlap. A negative value of β corresponds to stabilization, and the energies of the six orbitals are (lowest to highest): α + 2β, α + β, α + β, α − β, α − β, and α − 2β.28 The total energy of the three occupied orbitals is 6α + 8β, since there are two electrons in each orbital. The energy of an ordinary double bond is α + β, so that structure 1 or 2 has an energy of 6α + 6β and the resonance energy of benzene is 2β. Unfortunately, there is no convenient way to calculate the value of β from MO theory. Benzene is often given a value of β of ~ 18 kcal mol−1 (76 kJ mol−1); this number is one-half of the resonance energy calculated from heats of combustion or hydrogenation. Using ab initio calculations, bond resonance energies for many aromatic hydrocarbons other than benzene have been reported.29
Isodesmic and homodesmotic reactions are frequently used for the study of aromaticity from the energetic point of view.30 However, the energy of the reactions used experimentally, or in calculations, may reflect only the relative aromaticity of benzene and not its absolute aromaticity. New homodesmotic reactions based on radical systems predict an absolute aromaticity of 29.13 kcal mol−1 (121.9 kJ mol−1) for benzene and an absolute antiaromaticity (Sec. 2.K.ii) of 40.28 kcal mol−1 (168,5 kJ mol−1) for cyclobutadiene at the MP4(SDQ)/6-31G-(d,p) level.31
Compounds that exhibit delocalization are expected to have bond distances that lie between the values given in Table 1.5. This is certainly the case for benzene, since the carbon–carbon bond distance is 1.40 Å,32 which is between the 1.48 Å for an sp2–sp2 C–C single bond and the 1.32 Å of the sp2–sp2 C=C double bond.33