March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 2. Delocalized Chemical Bonding
2.G. Steric Inhibition of Resonance and the Influences of Strain
Rule 3 above states that all the atoms covered by delocalized electrons must lie in a plane or nearly so. Many examples are known where resonance is diminished or prevented because the atoms are sterically forced out of planarity. It is known that if para substituents are able to interact via the through-resonance mechanism, π-electron delocalization due to the substituent effects leads to an increase of stability.61 If the substituents are both electron donating, there is a significant decrease in stability.
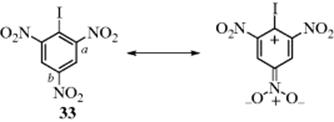
Bond lengths for the o- and p-nitro groups in picryl iodide (33) are quite different.62 Distance a in 33 is 1.45 Å, whereas b is 1.35 Å. This phenomenon can be explained if the oxygen atoms of the p-nitro group are in the plane of the ring and thus in resonance with it, so that b has partial double-bond character, while the oxygen atoms of the o-nitro groups are forced out of the plane by the large iodine atom. As discussed in Section 2.M, the difference in bond length is associated with hyperconjugative effects, represented by the canonical form.
Compound 34 is recognized as a Dewar-form of anthracene.63 Dewar benzene forms (e.g., benzvalene) (3) are recognized as possible valence isomers of benzene.64 Since 35 is the actual structure of anthracene, it is reasonable to ask if 34 is a canonical structure? The answer is no, because the 9,10 substituents prevent the system from being planar, so 34 is the actual structure of the molecule and it is not in resonance with forms like 35. This finding is a consequence of rule 2 (Sec. 2.E). In order for a 35-like structure to contribute to resonance in 34, the nuclei would have to be in the same positions in both forms. In anthracene itself (35), Dewar structures are thought to contribute to the structure, however.
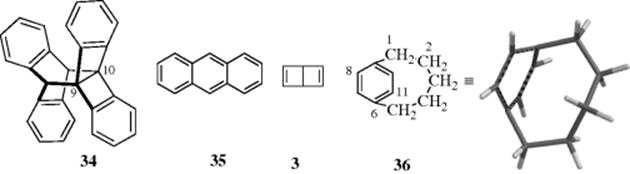
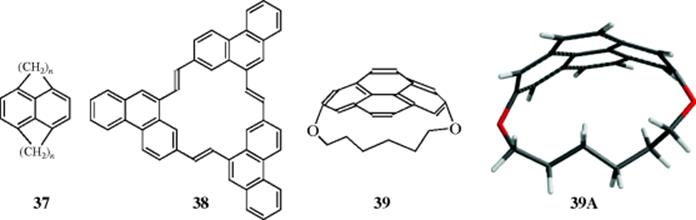
Even the benzene ring can be forced out of planarity.65 In [5]-paracyclophane66 (36) the presence of a short bridge (this is the shortest para bridge known for a benzene ring) forces the benzene ring to become boat shaped. The distortion in the benzene ring is apparent in the molecular model of 36 that is provided. The parent 36 has so far not proven stable enough for isolation, but a UV spectrum was obtained and showed that the benzene ring was aromatic, despite the distortion.67 The 8,11-dichloro analogue of 36 is a stable solid, and X-ray diffraction showed that benzene ring to be boat-shaped, with one end of the boat bending ~27° out of the plane, and the other ~12°.68This compound too is aromatic, as shown by UV and NMR spectra. [6]-Paracyclophanes are also bent,69 but in [7]-paracyclophanes the bridge is long enough so that the ring is only moderately distorted. Similarly, [n,m]paracyclophanes (37), where n and m are both 3 or less (the smallest yet prepared is [2.2]-paracyclophane),70 have bent (boat-shaped) benzene rings. All these compounds have properties that depart significantly from those of ordinary benzene compounds. Strained paracyclophanes exhibit both π- and σ-strain, and the effect of the two types of strain on the geometry is approximately additive.71 In “belt” cyclophane (38),72 the molecule has a pyramidal structure with C3 symmetry rather than the planar structure found in [18]annulene. 1,8-Dioxa[8](2,70-pyrenophane (39)73 is another severely distorted aromatic hydrocarbon (see 39A), in which the bridge undergoes rapid pseudo-rotation (Sec. 4.O.iv). A recent study showed that despite substantial changes in the hybridization of carbon atoms involving changes in the σ-electron structure of pyrenephane (e.g., 39), the aromaticity of the system decreases slightly and regularly upon increasing the bend angle θ from 0° to 109.2°.74 Heterocyclic paracyclophane analogues have been prepared, (e.g., the [2.n](2,5)pyridinophanes).75
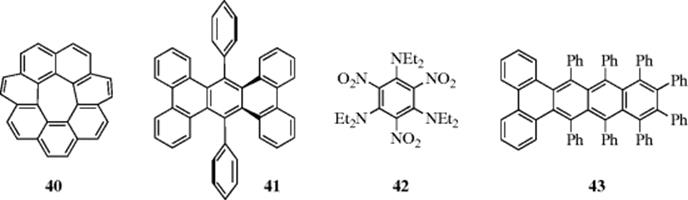
There are many examples of molecules in which benzene rings are forced out of planarity, including 7-circulene (40),76 9,8-diphenyltetrabenz[a,c,h,j]anthracene (41),77 and 4278 (see also, Sec. 4.Q.iv). These have been called tormented aromatic systems.79 The “record” for twisting an aromatic π-electron system appears to be 9,10,11,12,13,14,15,16-octaphenyldibenzo[a,c]naphthacene (43),80 which has an end-to-end twist of 105°. This was 1.5 times greater than that observed in any previous polyaromatic hydrocarbon. Perchlorotriphenylene has been reported in the literature and said to show severe molecular twisting, however, recent work suggests this molecule was not actually isolated, with perchlorofluorene-9-spirocyclohexa-2',5'-diene being formed instead.81 The X-ray structure of the linear [3]phenylene (benzo[3,4]cyclobuta[1,2-b]biphenylene, 44) has been obtained, and it shows a relatively large degree of bond alternation while the center distorts to a cyclic bis-(allyl) frame.82
It is also possible to fuse strained rings on benzene, which induces great strain on the benzene ring. In 45, the benzene ring is compressed by the saturated environment of the tetrahydropyran units and the strain leads to distortion of the benzene ring into a boat conformation.83
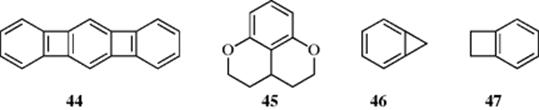
The term strain-induced bond localization was introduced in 1930 by Mills and Nixon84 and is commonly referred to as the Mills–Nixon effect (see Sec. 11.B.v). Ortho-fused aromatic compounds, (e.g., benzocyclopropene, 46), are known as cycloproparenes85 and are highly strained. Cyclopropabenzene (46) is a stable molecule with a strain energy of 68 kcal mol−1 (284.5 kJ mol−1),86 and the annellated bond is always the shortest, although in benzocyclobutene (47) the adjacent bond is the shortest.87 The bonds of annellation and those adjacent are strained. In cycloproparenes, there is the expectation of partial aromatic bond localization, with bond length alternation in the aromatic ring.88 When the bridging units are saturated, the benzene ring current is essentially unchanged, but annelation with one or more cyclobutadieno units disrupts the benzene ring current.89 The chemistry of the cycloproparenes is dominated by the influence of the high strain energy. When fused to a benzene ring, the bicyclo[1.1.0]butane unit also leads to strain-induced localization of aromatic π bonds.90