March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 2. Delocalized Chemical Bonding
2.L. Other Aromatic Compounds
Three additional types of aromatic compounds must be noted.
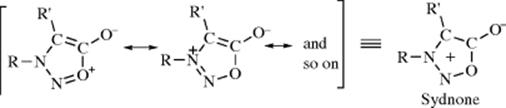
1. Mesoionic Compounds:376 These compounds cannot be satisfactorily represented by Lewis structures not involving charge separation. Most of them contain five-membered rings. The most common are the sydnones, stable aromatic compounds that undergo aromatic substitution when R' is hydrogen.
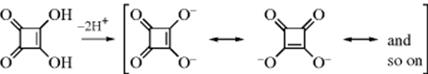
2. The Dianion of Squaric Acid.377 The stability of this system is illustrated by the fact that the pK1 of squaric acid378 is ~ 1.5 and the pK2 is ~ 3.5,379 which means that even the second proton is given up much more readily than the proton of acetic acid.380 The analogous three-,381 five-, and six-membered ring compounds are also known.382
3. Homoaromatic Compounds. When cyclooctatetraene is dissolved in concentrated H2SO4, a proton adds to one of the double bonds to form the homotropylium ion (137).383 In this species, an aromatic sextet is spread over seven carbons, as in the tropylium ion. The eighth carbon is an sp3 carbon and so cannot take part in the aromaticity. The NMR spectra show the presence of a diatropic ring current: Hb is found at δ = −0.3; Ha at 5.1 δ; H1 and H7 at 6.4 δ; H2–H6 at 8.5 δ. This ion is an example of a homoaromatic compound, generally defined as a compound that contains one or more384 sp3-hybridized carbon atoms in an otherwise conjugated cycle.385
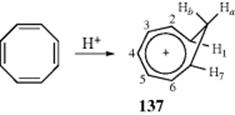
In order for the orbitals to overlap most effectively so as to close a loop, the sp3 atoms are forced to lie almost vertically above the plane of the aromatic atoms.386 In 137, Hb is directly above the aromatic sextet, and so is shifted far upfield in the NMR. Virtually all homoaromatic compounds so far discovered are ions, and the existence of homoaromatic character in uncharged systems387 has been questioned.388 However, neutral homoaromaticity in some heterocyclic compounds has been observed by replacing CH2 at C-2 in bicyclo[3.2.1]octa-3,6-diene with X = BH, AlH, Be, Mg, O, S, PH, NH (antihomoaromatic for X = BH, AlH, and Be; nonhomoaromatic for X = O, S, NH, PH); replacement of CH at C-3 in bicyclo[3.2.1]octa-3,6-dien-2-yl anion with PH, S, NH, O (homoaromatic for X = S, PH, NH, O); and replacement at C-2 and C-3 with N and O (homoaromatic).389 Homoaromatic ions of 2 and 10 electrons are also known.
New conceptual applications to 3D homoaromatic systems with cubane, dodecahedrane, and adamantane frameworks have been presented.390 This concept includes families of spherical homoaromatics with both two and eight mobile electrons. Each set has complete spherical homoaromaticity, that is, all the sp2 carbon atoms in a highly symmetrical framework are separated by one or two sp3-hybridized atoms.
4. Fullerenes. Fullerenes are a family of aromatic hydrocarbons391 based on the parent buckminsterfullerene (138; C60)392 that have a variety of very interesting properties.393 Derivatives of 138 are sometimes called buckyballs. Molecular orbital calculations show that “fullerene aromaticity lies within 2 kcal mol−1 (8.4 kJ mol−1) per carbon of a hypothetical ball of rolled up graphite.394 Fullerenes may exhibit what is known as spherical aromaticity (3D aromaticity),395 and the Hückel rule cannot be used for spherical systems (e.g., fullerenes). The 2(n + 1)2 rule was proposed by Hirsch et al,396 as the 3D analogue of the 4n + 2 rule for planar systems proposed by Hückel.397Heterofullerenes are also known.398
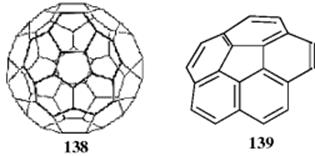
Buckybowls constitute another class of polynuclear aromatic hydrocarbons, and they are essentially fragments of 138. Corannulene (139)399 (also called 5-circulene), for example, is the simplest curved-surface hydrocarbon possessing a carbon framework that be identified with the buckminsterfullerene surface. It has been synthesized by Scott, et al.399 and several other groups.400 Corannulene is a flexible molecule, with a bowl–bowl inversion barrier of ~ 10–11 kcal mol−1 (41.8–46.0 kJ mol−1).401 Benzocorannulenes are known,402 and other bowl-shaped hydrocarbons include acenaphtho[3,2,1,8-ijklm]diindeno[4,3,2,1-cdef-1',2',3',4'pqra]triphenylene.403 The inversion barrier to buckybowl inversion has been lowered by such benzannelation of the rim.404 Other semibuckminsterfullerenes include C2v-C30H12 and C3-C30H12.399 Larger fullerenes include C60,C80, C84, and fullerenes are known that contain an endohedral metal (e.g., scandium or even Sc3N).405 Synthetic methods often generate mixtures of fullerenes that must be separated, as in the report of new methods for separating C84-fullerenes.406 A homofullerene has been prepared,407 and the azaacepentalenide anion, which is a bowl-shaped hetereocycle, has been prepared.408