March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th Edition (2013)
Part I. Introduction
Chapter 3. Bonding Weaker Than Covalent
The discussions in Chapters 1 and 2 focused on the structure of molecules as an aggregate of atoms in a distinct 3D arrangement, held together by bonds with energies on the order of 50–100 kcal mol−1 (200–400 kJ mol−1). There are also very weak attractive forces between molecules, on the order of a few tenths of a kilocalorie per mole. These forces, called van der Waals forces,1 are caused by electrostatic attractions, such as those between dipole and dipole, or induced dipole and induced dipole, and are responsible for liquefaction of gases at sufficiently low temperatures. The bonding discussed in this chapter has energies on the order of 2–10 kcal mol−1 (9–40 kJ/mol−1), which is intermediate between the bond orders given above, and produces clusters of molecules. Compounds will also be discussed in which portions of molecules are held together without any attractive forces at all.
3.A. Hydrogen Bonding2
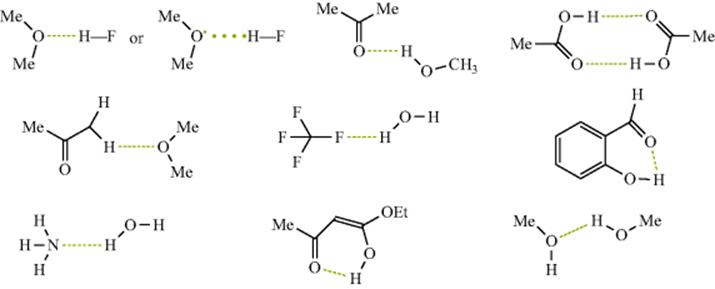
A hydrogen bond is less than a covalent bond, but it is an attractive force between a functional group A–H and an atom or group of atoms B in the same or a different molecule.3 With exceptions to be noted later, hydrogen bonds are assumed to form only when A is oxygen, nitrogen, or fluorine and when B is oxygen, nitrogen, or fluorine.4 The ability of functional groups to act as hydrogen-bond acids and bases can be obtained from either equilibrium constants for 1:1 hydrogen bonding or overall hydrogen-bond constants.4 There are so-called unconventional hydrogen bonds, particularly with organometallic complexes and transition metal or main group hydrides.5 This chapter will largely ignore such compounds. In normal hydrogen bonds, to oxygen or nitrogen, oxygen may be singly or doubly bonded and the nitrogen singly, doubly, or triply bonded. In structures, hydrogen bonds are usually represented by dotted or dashed lines, as shown in the following examples:
Hydrogen bonds can exist in solid6 and liquid phases, and in solution.7 The efficacy of many organic reactions that will be discussed in later chapters is due to the use of an aqueous media,8 which is due in part, to the hydrogen-bonding nature of such media.9 Even in the gas phase, compounds that form particularly strong hydrogen bonds may remain associated.10 Acetic acid, for example, exists in the gas phase as a dimer, except at very low pressures.11 In the liquid phase and in solution, hydrogen bonds rapidly form and break. The mean lifetime of the NH3![]() H2O bond is 2 × 10-12 s, for example.12 Except for a few very strong hydrogen bonds13 (e.g., the FH
H2O bond is 2 × 10-12 s, for example.12 Except for a few very strong hydrogen bonds13 (e.g., the FH![]() F− bond) which has an energy of ~50 kcal mol-1 or 210 kJ mol-1, the strongest hydrogen bonds are those connecting one carboxylic acid with another. The energies of these bonds are in the range of 6–8 kcal mol-1 or 25–30 kJ mol-1 (for carboxylic acids, this refers to the energy of each bond). In general, short contact hydrogen bonds between fluorine and HO or NH are rare.14 Other OH
F− bond) which has an energy of ~50 kcal mol-1 or 210 kJ mol-1, the strongest hydrogen bonds are those connecting one carboxylic acid with another. The energies of these bonds are in the range of 6–8 kcal mol-1 or 25–30 kJ mol-1 (for carboxylic acids, this refers to the energy of each bond). In general, short contact hydrogen bonds between fluorine and HO or NH are rare.14 Other OH![]() O and NH
O and NH![]() N bonds15 have energies of 3–6 kcal mol-1 (12–25 kJ mol-1). The intramolecular O–H
N bonds15 have energies of 3–6 kcal mol-1 (12–25 kJ mol-1). The intramolecular O–H![]() N hydrogen bond in hydroxy-amines is also rather strong.16
N hydrogen bond in hydroxy-amines is also rather strong.16
To a first approximation, the strength of a hydrogen bond will increase with increasing acidity of A–H17 and basicity of B, but the parallel is far from exact.18 A quantitative measure of the strengths of hydrogen bonds has been established, involving the use of an α scale to represent hydrogen-bond donor acidities and a β scale for hydrogen-bond acceptor basicities.19 The use of the β scale, along with another parameter (ξ), allows hydrogen-bond basicities to be related to proton-transfer basicities (pK values).20 A database has been developed to locate all possible occurrences of bimolecular cyclic hydrogen-bond motifs in the Cambridge Structural Database,21 and donor–acceptor, as well as polarity parameters, have been calculated for hydrogen-bonding solvents.22 Bickelhaupt and co-workers22 has stated that hydrogen bonds (X–H![]() Y) have significant covalent interactions that stem from donor–acceptor orbital interactions between the lone-pair electrons of Y and the empty σ∗ acceptor orbital of X–H, so they are not predominantly electrostatic phenomena.
Y) have significant covalent interactions that stem from donor–acceptor orbital interactions between the lone-pair electrons of Y and the empty σ∗ acceptor orbital of X–H, so they are not predominantly electrostatic phenomena.
When two compounds whose molecules form hydrogen bonds with each other are both dissolved in water, the hydrogen bond between the two molecules is usually greatly weakened or completely removed.23 This finding means that the molecules generally form hydrogen bonds with the water molecules (intermolecular) rather than with each other (intramolecular), presumably because the water molecules are present in such great numbers. In amides, the oxygen atom is the preferred site of protonation or complexation with water.24 In the case of dicarboxylic acids, arguments have been presented that there is little or no evidence for strong hydrogen bonding in aqueous solution,25although other studies concluded that strong, intramolecular hydrogen bonding can exist in aqueous acetone solutions (0.31 mole-fraction water) of hydrogen maleate and hydrogen cis-cyclohexane-1,2-dicarboxylate.26
Many studies have been made of the geometry of hydrogen bonds,27 and in most (though not all) cases the evidence shows that the hydrogen is on or near the straight line formed by A and B.28 This is true both in the solid state (where X-ray crystallography and neutron diffraction have been used to determine structures),29 and in solution.30 It is significant that the vast majority of intramolecular hydrogen bonding occurs where six-membered rings(counting the hydrogen as one of the six) can be formed, in which linearity of the hydrogen bond is geometrically favorable. Intramolecular hydrogen bonding is much rarer in five-membered rings, where linearity is usually not favored (although it is known). A novel nine-membered intramolecular hydrogen bond has been reported.31
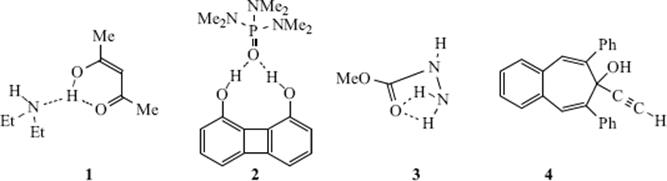
In certain cases X-ray crystallography has shown that a single H–A can form simultaneous hydrogen bonds with two B atoms (bifurcated or three-center hydrogen bonds). An example is an adduct (1) formed from pentane-2,4-dione in its enol form (Sec. 2.N.i) and diethylamine. In 1, the O–H hydrogen simultaneously bonds32 to an O and an N, where the N–H hydrogen forms a hydrogen bond with the O of another pentane-2,4-dione molecule.33 On the other hand, the B atom (in this case oxygen) forms simultaneous hydrogen bonds with two A![]() H hydrogens in the adduct (2) formed from 1,8-biphenylenediol and hexamethylphosphoramide (HMPA),34 Another such case is found in methyl hydrazine carboxylate (3).35 Except for the special case of FH
H hydrogens in the adduct (2) formed from 1,8-biphenylenediol and hexamethylphosphoramide (HMPA),34 Another such case is found in methyl hydrazine carboxylate (3).35 Except for the special case of FH![]() F− bonds (see above), the hydrogen is not equidistant between A and B. For example, the O–H distance in ice is 0.97 Å, while the H
F− bonds (see above), the hydrogen is not equidistant between A and B. For example, the O–H distance in ice is 0.97 Å, while the H![]() O distance is 1.79 Å.36A theoretical study of the vinyl alcohol–vinyl alcoholate system (i.e., an enol–enolate anion system) concluded the hydrogen bonding is strong but asymmetric.37 The hydrogen bond in the enol of malonaldehyde, in organic solvents, is asymmetric with the hydrogen atom closer to the basic oxygen atom.38 There is evidence, however, that symmetrical hydrogen bonds to carboxylates should be regarded as two-center rather than three-center hydrogen bonds since the criteria traditionally used to infer three-center hydrogen bonding are inadequate for carboxylates.39 There is also an example of cooperative hydrogen bonding [O–H
O distance is 1.79 Å.36A theoretical study of the vinyl alcohol–vinyl alcoholate system (i.e., an enol–enolate anion system) concluded the hydrogen bonding is strong but asymmetric.37 The hydrogen bond in the enol of malonaldehyde, in organic solvents, is asymmetric with the hydrogen atom closer to the basic oxygen atom.38 There is evidence, however, that symmetrical hydrogen bonds to carboxylates should be regarded as two-center rather than three-center hydrogen bonds since the criteria traditionally used to infer three-center hydrogen bonding are inadequate for carboxylates.39 There is also an example of cooperative hydrogen bonding [O–H![]() C≡C–H
C≡C–H![]() Ph] in crystalline 2-ethynyl-6,8-diphenyl-7H-benzocyclohepten-7-ol (4).40 Related to this discussion is the work that showed the hydrogen bond radii for OH, NH, and acidic CH groups to be 0.60 ± 0.15, 0.76 ± 0.15, and 1.10 ± 0.20 Å, respectively.41
Ph] in crystalline 2-ethynyl-6,8-diphenyl-7H-benzocyclohepten-7-ol (4).40 Related to this discussion is the work that showed the hydrogen bond radii for OH, NH, and acidic CH groups to be 0.60 ± 0.15, 0.76 ± 0.15, and 1.10 ± 0.20 Å, respectively.41
Hydrogen bonding has been detected in many ways, including measurements of dipole moments, solubility behavior, freezing-point lowering, and heats of mixing, but one important way is by the effect of the hydrogen bond on IR spectroscopy.42 The IR frequencies of functional groups (e.g., O–H or C=O) are shifted when the group is hydrogen bonded. Hydrogen bonding always moves the peak toward lower frequencies, for both the A–H and the B groups, though the shift is greater for the former. For example, a free OH group of an alcohol or phenol absorbs at ~ 3590–3650 cm−1, and a hydrogen-bonded OH group is found ~ 50–100 cm-1 lower.43 In many cases, in dilute solution, there is partial hydrogen bonding, that is, some OH groups are free and some are hydrogen bonded. In such cases, two peaks appear.
Infrared spectroscopy can also distinguish between inter- and intramolecular hydrogen bonding, since intermolecular peaks are intensified by an increase in concentration while intramolecular peaks are unaffected. Other types of spectra that have been used for the detection of hydrogen bonding include Raman, electronic,44 and NMR.45 Since hydrogen bonding involves a rapid movement of protons from one atom to another, NMR records an average value that is often broadened. Hydrogen bonding can be detected because it usually produces a chemical shift to a lower field. For example, carboxylic acid–carboxylate systems arising from either mono- or diacids generally exhibit a downfield resonance (16–22 ppm), which indicates “strong” hydrogen bonding in anhydrous, aprotic solvents.46 Hydrogen bonding changes with temperature and concentration, and comparison of spectra taken under different conditions also serves to detect and measure it. As with IR spectra, intramolecular hydrogen bonding in the NMR can be distinguished from intermolecular by its constancy when the concentration is varied. The spin–spin coupling constant across a hydrogen bond, obtained by NMR studies, has been shown to provide a “fingerprint” for hydrogen-bond type.47 Indeed, the determination of ![]() correlates with the strength of the hydrogen bonds formed by an alcohol.48
correlates with the strength of the hydrogen bonds formed by an alcohol.48
Hydrogen bonds are important because of the effects they have on the properties of compounds, among them:
1. Intermolecular hydrogen bonding raises boiling points and frequently melting points.
2. If hydrogen bonding is possible between solute and solvent, this greatly increases solubility and often results in large or even infinite solubility where none would otherwise be expected.
3. Hydrogen bonding causes lack of ideality in gas and solution laws.
4. As previously mentioned, hydrogen bonding changes spectral absorption positions.
5. Hydrogen bonding, especially the intramolecular variety, changes many chemical properties. For example, it is responsible for the large amount of enol present in certain tautomeric equilibria (see Sec. 2.N). Also, by influencing the conformation of molecules (see Chapter 4), it often plays a significant role in determining reaction rates.49 Hydrogen bonding is also important in maintaining the 3D structures of protein and nucleic acid molecules.
Besides oxygen, nitrogen, and fluorine, there is evidence that weaker hydrogen bonding exists in other systems.50 Although many searches have been made for hydrogen bonding where A is carbon,51 only three types of C–H bonds have been found that are acidic enough to form weak hydrogen bonds.52 These are found in terminal alkynes (RC![]() CH),53 chloroform and some other halogenated alkanes, and HCN. Sterically unhindered C–H groups (CHCl3, CH2Cl2, RC
CH),53 chloroform and some other halogenated alkanes, and HCN. Sterically unhindered C–H groups (CHCl3, CH2Cl2, RC![]() CH) form short contact hydrogen bonds with carbonyl acceptors, where there is a significant preference for coordination with the conventional carbonyl lone-pair direction.54 Weak hydrogen bonds are formed by compounds containing S–H bonds.55 There has been much speculation regarding other possibilities for B. There is evidence that Cl can form weak hydrogen bonds,56 but Br and I form very weak bonds if at all.57 However, the ions Cl−, Br−, and I− form hydrogen bonds that are much stronger than those of the covalently bonded atoms.58 As noted above, the FH
CH) form short contact hydrogen bonds with carbonyl acceptors, where there is a significant preference for coordination with the conventional carbonyl lone-pair direction.54 Weak hydrogen bonds are formed by compounds containing S–H bonds.55 There has been much speculation regarding other possibilities for B. There is evidence that Cl can form weak hydrogen bonds,56 but Br and I form very weak bonds if at all.57 However, the ions Cl−, Br−, and I− form hydrogen bonds that are much stronger than those of the covalently bonded atoms.58 As noted above, the FH![]() F− bond is especially strong. In this case, the hydrogen is equidistant from the fluorine atoms.59 Similarly, a sulfur atom55 can be the B component (A
F− bond is especially strong. In this case, the hydrogen is equidistant from the fluorine atoms.59 Similarly, a sulfur atom55 can be the B component (A![]() B) in weak hydrogen bonds,60 but the −SH ion forms much stronger bonds.61 There are theoretical studies of weak hydrogen bonding.62 Hydrogen bonding has been directly observed (by NMR and IR) between a negatively charged carbon (see carbanions in Chapter 5) and an OH group in the same molecule.63 Isocyanides (R–+N
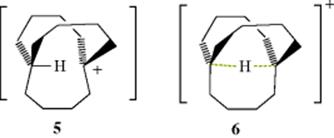
B) in weak hydrogen bonds,60 but the −SH ion forms much stronger bonds.61 There are theoretical studies of weak hydrogen bonding.62 Hydrogen bonding has been directly observed (by NMR and IR) between a negatively charged carbon (see carbanions in Chapter 5) and an OH group in the same molecule.63 Isocyanides (R–+N![]() C−) constitute another type of molecule in which carbon is the B component that forms a rather strong hydrogen bond.64 There is evidence that double and triple bonds, aromatic rings,65 and even cyclopropane rings66 may be the B component of hydrogen bonds, but these bonds are very weak. An interesting case is that of the in-bicyclo[4.4.4]-1-tetradecyl cation 5 (see out–in isomerism, Sec. 4.L The NMR and IR spectra show that the actual structure of this ion is 6, in which both the A and the B component of the hydrogen bond is a carbon.67 These are sometimes called 3-center-2-electron C–H–C bonds.68 A technique called generalized population analysis has been developed to study this type of multicenter bonding.69
C−) constitute another type of molecule in which carbon is the B component that forms a rather strong hydrogen bond.64 There is evidence that double and triple bonds, aromatic rings,65 and even cyclopropane rings66 may be the B component of hydrogen bonds, but these bonds are very weak. An interesting case is that of the in-bicyclo[4.4.4]-1-tetradecyl cation 5 (see out–in isomerism, Sec. 4.L The NMR and IR spectra show that the actual structure of this ion is 6, in which both the A and the B component of the hydrogen bond is a carbon.67 These are sometimes called 3-center-2-electron C–H–C bonds.68 A technique called generalized population analysis has been developed to study this type of multicenter bonding.69
A weak (~1.5 kcal mol-1, 6.3 kJ mol-1) and rare C–H![]() O=C hydrogen bond has been reported in a class of compounds known as a [6]semirubin (a dipyrrinone).70 There is also evidence for a C–H
O=C hydrogen bond has been reported in a class of compounds known as a [6]semirubin (a dipyrrinone).70 There is also evidence for a C–H![]() N/CH
N/CH![]() OH bond in the crystal structures of α,β-unsaturated ketones carrying a terminal pyridine subunit,71 and for R3N+–C–H
OH bond in the crystal structures of α,β-unsaturated ketones carrying a terminal pyridine subunit,71 and for R3N+–C–H![]() O=C hydrogen bonding.72
O=C hydrogen bonding.72
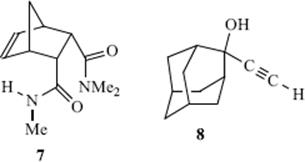
Deuterium also forms hydrogen bonds; in some systems these seem to be stronger than the corresponding hydrogen bonds; in others, weaker.73 Weak hydrogen bonds can be formed between a π bond (from both alkenes and aromatic compounds) and an appropriate hydrogen. For example, IR data in dilute dichloromethane suggests that the predominant conformation for bis-(amide) (7) contains an N–H![]() π hydrogen bond involving the C=C unit.74 The strength of an intramolecular π-facial hydrogen bond between an NH group and an aromatic ring in chloroform has been estimated to have a lower limit of −4.5 ± 0.5 kcal mol-1 (−18.8 kJ mol−1).75 A neutron diffraction study of crystalline 2-ethynyladamantan-2-ol (8) shows the presence of an unusual O–H
π hydrogen bond involving the C=C unit.74 The strength of an intramolecular π-facial hydrogen bond between an NH group and an aromatic ring in chloroform has been estimated to have a lower limit of −4.5 ± 0.5 kcal mol-1 (−18.8 kJ mol−1).75 A neutron diffraction study of crystalline 2-ethynyladamantan-2-ol (8) shows the presence of an unusual O–H![]() π hydrogen bond, which is short and linear, as well as the more common O–H
π hydrogen bond, which is short and linear, as well as the more common O–H![]() O and C–H
O and C–H![]() O hydrogen bonds.76
O hydrogen bonds.76